
Write a note on Friedel Crafts acylation.
Answer
594k+ views
Hint: The two primary types of Friedel-Crafts reactions are the alkylation and acylation reactions. Friedel craft acylation is a type of an organic reaction in which an acyl group $( - COR)$is attached to a benzene ring.
Complete answer:
As we know, Friedel craft acylation is a type of an organic reaction in which an acyl group is attached to a benzene ring. This can be easily understood with the help of an example: When benzene is reacted with an acyl halide in the presence of anhydrous aluminium chloride, it gives acyl benzene as a product. And it is a type of electrophilic substitution reaction, electrophiles, as we know, are electron-loving. This can be referred to in other words as electron-deficient species and thus can accept an electron pair from electron-rich species. Some of the examples are carbocations and carbonyl compounds, and the term substitution refers to the removal of a hydrogen atom and is replaced by group or an atom in this case hydrogen is replaced by an acyl group. The reaction for the above-mentioned example is as follows:
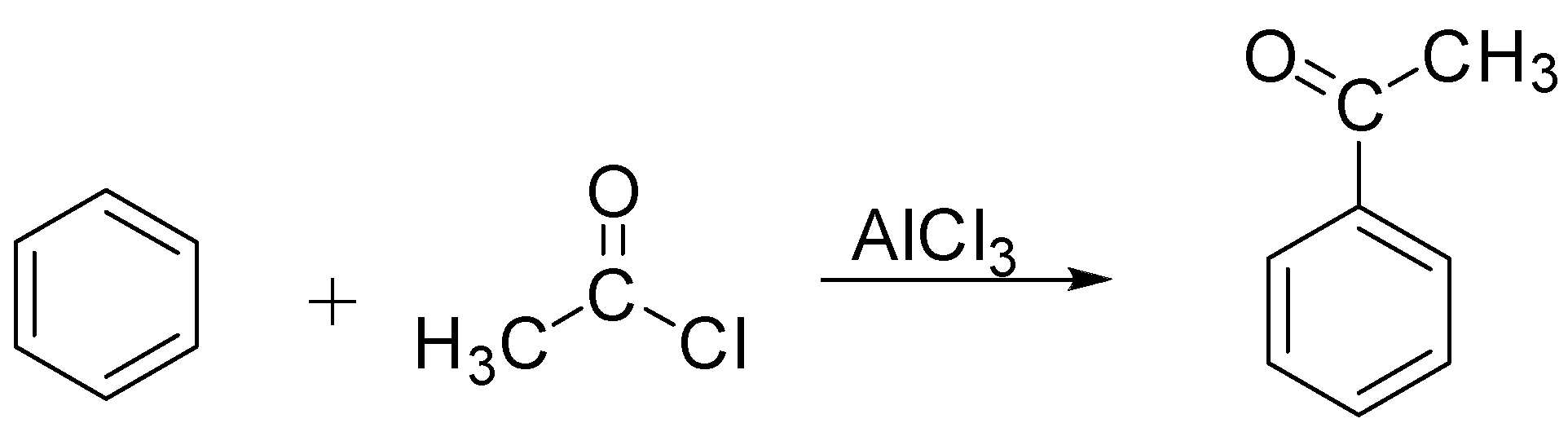
Here Acyl group is an electrophile as there is a formation of partial positive charge on the carbon atom of the acyl group. Acylation reactions can also be carried out with acetic anhydride as a substrate in the presence of Anhydrous $AlC{l_3}$ .
Note:
There are many types of Friedel Crafts reaction but a student can confuse between the alkylation and the acylation reaction these are quite a similar type of reaction as in Alkylation there is a substitution of hydrogen by an alkyl group and in acylation, there is a substitution of hydrogen by an acyl group.
Complete answer:
As we know, Friedel craft acylation is a type of an organic reaction in which an acyl group is attached to a benzene ring. This can be easily understood with the help of an example: When benzene is reacted with an acyl halide in the presence of anhydrous aluminium chloride, it gives acyl benzene as a product. And it is a type of electrophilic substitution reaction, electrophiles, as we know, are electron-loving. This can be referred to in other words as electron-deficient species and thus can accept an electron pair from electron-rich species. Some of the examples are carbocations and carbonyl compounds, and the term substitution refers to the removal of a hydrogen atom and is replaced by group or an atom in this case hydrogen is replaced by an acyl group. The reaction for the above-mentioned example is as follows:

Here Acyl group is an electrophile as there is a formation of partial positive charge on the carbon atom of the acyl group. Acylation reactions can also be carried out with acetic anhydride as a substrate in the presence of Anhydrous $AlC{l_3}$ .
Note:
There are many types of Friedel Crafts reaction but a student can confuse between the alkylation and the acylation reaction these are quite a similar type of reaction as in Alkylation there is a substitution of hydrogen by an alkyl group and in acylation, there is a substitution of hydrogen by an acyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




