
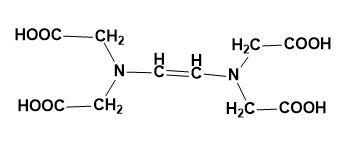
The correct structure of ethylenediaminetetraacetic acid (EDTA) is:
(A)
(B)
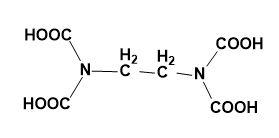
(C)
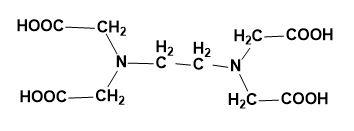
(D)
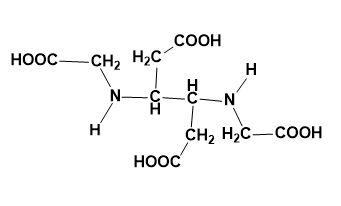




Answer
572.1k+ views
Hint:. EDTA (ethylenediaminetetraacetic acid) is a tetraprotic acid. As the name suggests, the structure will consist of ethane groups attached to the two amine groups which will result in the formation of ethylene diamine. Also, the four hydrogen atoms are replaced by the acetic acid groups resulting in complex structure.
- The molecular formula for EDTA is ${{C}_{10}}{{H}_{16}}{{N}_{2}}{{O}_{8}}$ .
Complete step by step answer:
Let us know about EDTA before determining its structure;
EDTA- Ethylenediaminetetraacetic acid is a complexing agent. It is a colourless and water-soluble compound in its salt form. It is a hexadentate ligand.
Structure- EDTA has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. The ethane is attached to two amine groups making it to be called as ethylene diamine. Then the two hydrogens from the amine (each) are replaced by the acetic acid group which makes EDTA possible.
The structure of EDTA is:
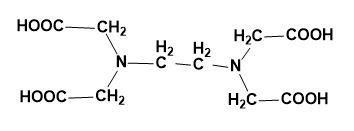
So, the correct answer is “Option C”.
Note: Do note that EDTA is an acid so it favours reactions in basic medium. It is actually a polyprotic acid as ethylenediaminetetraacetic acid (abbreviated as EDTA), can add two more hydrogens on the two nitrogen of the amine group.
- The molecular formula for EDTA is ${{C}_{10}}{{H}_{16}}{{N}_{2}}{{O}_{8}}$ .
Complete step by step answer:
Let us know about EDTA before determining its structure;
EDTA- Ethylenediaminetetraacetic acid is a complexing agent. It is a colourless and water-soluble compound in its salt form. It is a hexadentate ligand.
Structure- EDTA has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. The ethane is attached to two amine groups making it to be called as ethylene diamine. Then the two hydrogens from the amine (each) are replaced by the acetic acid group which makes EDTA possible.
The structure of EDTA is:

So, the correct answer is “Option C”.
Note: Do note that EDTA is an acid so it favours reactions in basic medium. It is actually a polyprotic acid as ethylenediaminetetraacetic acid (abbreviated as EDTA), can add two more hydrogens on the two nitrogen of the amine group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




