
Which one of the following reactions is consistent with Markovnikov's rule?
(A) ${{(C{{H}_{3}})}_{2}}CH-CH=C{{H}_{2}}+{{H}_{2}}O\to {{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}-C{{H}_{2}}-OH$
(B) $C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}+{{H}_{2}}O\to C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-OH$
(C) $C{{H}_{3}}CH=CHC{{H}_{3}}+{{H}_{2}}O\to C{{H}_{3}}C{{H}_{2}}-CH(C{{H}_{3}})-OH$
(D) $C{{H}_{2}}=CHC{{H}_{3}}+{{H}_{2}}O\to {{H}_{3}}C-CH(C{{H}_{3}})-OH$
Answer
592.2k+ views
Hint: This reaction follows the Markovnikov rule in the synthesis of alkanols. This rule is one of the important rules for the prediction of the electrophilic addition reaction of unsymmetrical alkenes in organic chemistry. This rule mechanism mainly depends on the stability of carbocation and its structure also predicts the product conformational structure.
Complete answer:
According to Markovnikov’s rule, in the given reaction alcohols from alkenes add water in the presence of acid. Let’s see out of four reactions, which one obeys the Markovnikov’s rule.
(A) \[{{(C{{H}_{3}})}_{2}}CH-CH=C{{H}_{2}}+{{H}_{2}}O\to {{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}-C{{H}_{2}}-OH\]
In the above reaction, there are two intermediates formed.
\[{{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}-C{{H}_{2}}^{+}\] --(1A)
\[{{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}^{+}-C{{H}_{3}}\] --(1B)
1A is primary carbocation and 1B is secondary carbocation which is more stable than 1A.
But the given equation product was formed by intermediate 1A, so this reaction does not obey Markovnikov’s rule.
(B) \[C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}+{{H}_{2}}O\to C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-OH\]
In the above reaction, there are two intermediates formed.
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}^{+}\] ---(2A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}^{+}}-C{{H}_{3}}\] --(2B)
2A is primary carbocation and 2B is secondary carbocation which is more stable than 2A.
But the given equation product was formed by an intermediate 2A reaction with hydroxyl ion. so this reaction does not obey Markovnikov’s rule.
(C) \[C{{H}_{3}}CH=CHC{{H}_{3}}+{{H}_{2}}O\to C{{H}_{3}}C{{H}_{2}}-CH(C{{H}_{3}})-OH\]
This is a symmetrical alkene named as 2-butene. Markovnikov’s rule does not apply for Symmetrical alkene.
(D) \[C{{H}_{2}}=CHC{{H}_{3}}+{{H}_{2}}O\to {{H}_{3}}C-CH(C{{H}_{3}})-OH\]
This alkene is named prop-1-ene. If this alkene reaction with ${{H}^{+}}$ forms two intermediate carbocations.
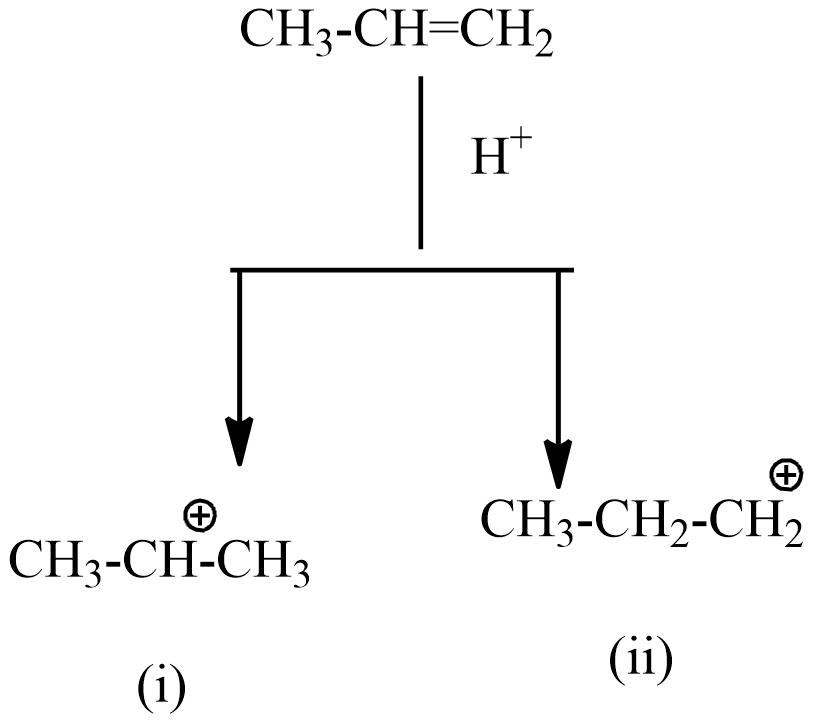
The intermediate (i) is a secondary carbocation which is more stable than intermediate (i).
Hence the intermediate (i) reacts with $O{{H}^{-}}$ , forms the below product.
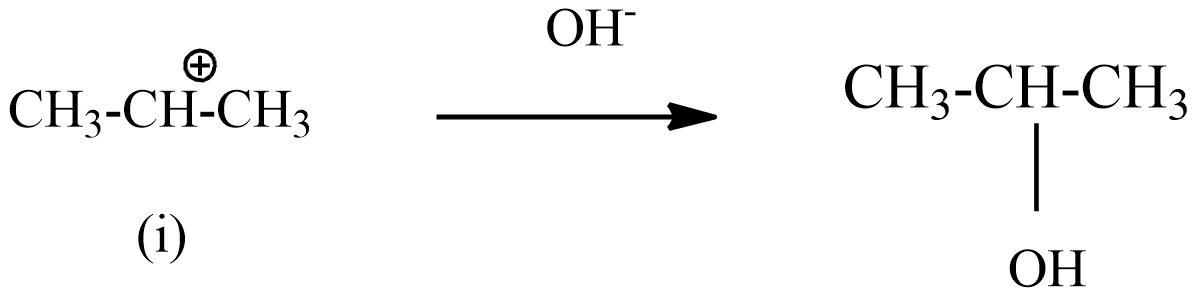
Hence, this reaction obeys Markovnikov’s rule.
So, the correct answer is “Option D”.
Note: Markovnikov’s rule does not apply to symmetrical alkenes. Especially ethene is symmetrical alkene, this rule does not apply. Because there is only one product from the electrophilic addition of ${{H}_{2}}O$ to ethene. There is an AntiMarkovnikov’s rule which includes that hydrogen atoms are attached to the carbon atom with the least hydrogen substituents. This rule explains the opposite of Markovnikov’s rule.
Complete answer:
According to Markovnikov’s rule, in the given reaction alcohols from alkenes add water in the presence of acid. Let’s see out of four reactions, which one obeys the Markovnikov’s rule.
(A) \[{{(C{{H}_{3}})}_{2}}CH-CH=C{{H}_{2}}+{{H}_{2}}O\to {{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}-C{{H}_{2}}-OH\]
In the above reaction, there are two intermediates formed.
\[{{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}-C{{H}_{2}}^{+}\] --(1A)
\[{{(C{{H}_{3}})}_{2}}CH-C{{H}_{2}}^{+}-C{{H}_{3}}\] --(1B)
1A is primary carbocation and 1B is secondary carbocation which is more stable than 1A.
But the given equation product was formed by intermediate 1A, so this reaction does not obey Markovnikov’s rule.
(B) \[C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}+{{H}_{2}}O\to C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-OH\]
In the above reaction, there are two intermediates formed.
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}^{+}\] ---(2A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}^{+}}-C{{H}_{3}}\] --(2B)
2A is primary carbocation and 2B is secondary carbocation which is more stable than 2A.
But the given equation product was formed by an intermediate 2A reaction with hydroxyl ion. so this reaction does not obey Markovnikov’s rule.
(C) \[C{{H}_{3}}CH=CHC{{H}_{3}}+{{H}_{2}}O\to C{{H}_{3}}C{{H}_{2}}-CH(C{{H}_{3}})-OH\]
This is a symmetrical alkene named as 2-butene. Markovnikov’s rule does not apply for Symmetrical alkene.
(D) \[C{{H}_{2}}=CHC{{H}_{3}}+{{H}_{2}}O\to {{H}_{3}}C-CH(C{{H}_{3}})-OH\]
This alkene is named prop-1-ene. If this alkene reaction with ${{H}^{+}}$ forms two intermediate carbocations.

The intermediate (i) is a secondary carbocation which is more stable than intermediate (i).
Hence the intermediate (i) reacts with $O{{H}^{-}}$ , forms the below product.

Hence, this reaction obeys Markovnikov’s rule.
So, the correct answer is “Option D”.
Note: Markovnikov’s rule does not apply to symmetrical alkenes. Especially ethene is symmetrical alkene, this rule does not apply. Because there is only one product from the electrophilic addition of ${{H}_{2}}O$ to ethene. There is an AntiMarkovnikov’s rule which includes that hydrogen atoms are attached to the carbon atom with the least hydrogen substituents. This rule explains the opposite of Markovnikov’s rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




