
Which one of the following acids does not exhibit optical isomerism?
(A) Lactic acid
(B) Tartaric acid
(C) Maleic acid
(D) $\alpha -$amino acids
Answer
566.4k+ views
Hint: For determining which of the acids does not exhibit optical isomerism, we know that only those compounds exhibit optical isomerism, which has a chiral centre or absence of symmetry elements.
Complete step by step solution:
We have been provided with acids: Lactic acid, Tartaric acid, Maleic acid and $\alpha -$amino acids,
We need to tell which one of them would exhibit optical isomerism,
So, for that:
First acid we have lactic acid:
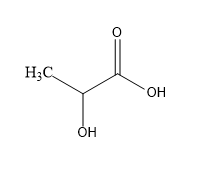
So, in lactic acid one chiral centre is present so it is optically active.
Next, we have Tartaric acid:
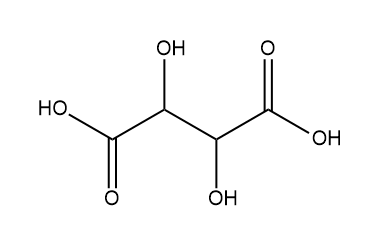
So, in tartaric acid two chiral centres are present so it is optically active.
Next, we have Maleic acid:
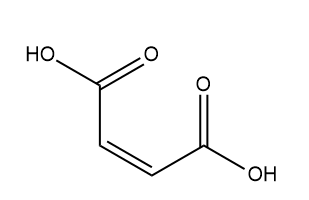
So, Maleic acid does not have any chiral centre, so it does not exhibit optical isomerism.
Last one we have $\alpha -$amino acid:
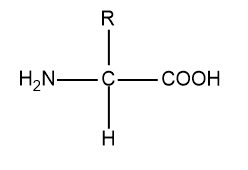
So, in $\alpha -$amino acid one chiral centre is present so it is optically active.
So, we can say that Maleic acid would not exhibit optical isomerism.
Therefore, we can conclude that option (C) is correct.
Note: Optical isomers are important because two isomers can have the same chemical formula, but have different chemical structures. And these structures contribute to the properties of the molecule.
Complete step by step solution:
We have been provided with acids: Lactic acid, Tartaric acid, Maleic acid and $\alpha -$amino acids,
We need to tell which one of them would exhibit optical isomerism,
So, for that:
First acid we have lactic acid:

So, in lactic acid one chiral centre is present so it is optically active.
Next, we have Tartaric acid:

So, in tartaric acid two chiral centres are present so it is optically active.
Next, we have Maleic acid:

So, Maleic acid does not have any chiral centre, so it does not exhibit optical isomerism.
Last one we have $\alpha -$amino acid:

So, in $\alpha -$amino acid one chiral centre is present so it is optically active.
So, we can say that Maleic acid would not exhibit optical isomerism.
Therefore, we can conclude that option (C) is correct.
Note: Optical isomers are important because two isomers can have the same chemical formula, but have different chemical structures. And these structures contribute to the properties of the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




