
Which one is not a glyceride?
A. fat
B. oil
C. phospholipid
D. soap
Answer
578.4k+ views
Hint: We should know what glyceride is to be able to eliminate the options and decide which of the following is not a glyceride. Glycerides are the esters formed by glycerol and fatty acids.
Complete step by step solution:
-Esters are the organic compounds with the functional group –COOR. They are formed as a result of the elimination reaction between the carboxylic acids and alcohols. Water is eliminated from the reaction to form the easters.
-The dehydration reaction between the carboxylic acids and alcohols to form esters can be shown as
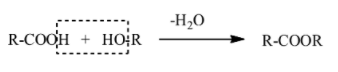
-The R group in fatty acids is very complex in nature. Glyceride is made from this and glycerol which has the structure as
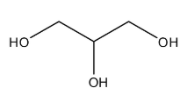
-As there are 3 different hydroxyl groups present in a glycerol, it can be esterified with three different or same fatty acids forming glycerides which will eventually have a very complex structure. They can form mono, di and triglycerides.
-Vegetable oils and animal fats contain these complex structures of glycerides. They are usually triglycerides only but can be easily broken down into mono and di-glycerides by the action of certain enzymes like lipases.
-So, fats, oils and phospholipids, all can form glycerides while soap is not a glyceride as it does not contain these groups. It has only one acidic hydrogen which is removed by the action of base and forms compounds of type $R-CO{{O}^{-}}$ .
Therefore the correct option is D.
Note: The reaction between acids and bases to form soap and water is called neutralization reaction because the reaction neutralizes the acidity of the reaction. Acids have very low pH and bases have high pH. But the soaps are neither acidic nor basic.
Complete step by step solution:
-Esters are the organic compounds with the functional group –COOR. They are formed as a result of the elimination reaction between the carboxylic acids and alcohols. Water is eliminated from the reaction to form the easters.
-The dehydration reaction between the carboxylic acids and alcohols to form esters can be shown as
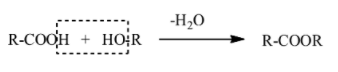
-The R group in fatty acids is very complex in nature. Glyceride is made from this and glycerol which has the structure as

-As there are 3 different hydroxyl groups present in a glycerol, it can be esterified with three different or same fatty acids forming glycerides which will eventually have a very complex structure. They can form mono, di and triglycerides.
-Vegetable oils and animal fats contain these complex structures of glycerides. They are usually triglycerides only but can be easily broken down into mono and di-glycerides by the action of certain enzymes like lipases.
-So, fats, oils and phospholipids, all can form glycerides while soap is not a glyceride as it does not contain these groups. It has only one acidic hydrogen which is removed by the action of base and forms compounds of type $R-CO{{O}^{-}}$ .
Therefore the correct option is D.
Note: The reaction between acids and bases to form soap and water is called neutralization reaction because the reaction neutralizes the acidity of the reaction. Acids have very low pH and bases have high pH. But the soaps are neither acidic nor basic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




