
Which one is a wrong statement?
A.The electronic configuration of N atom is
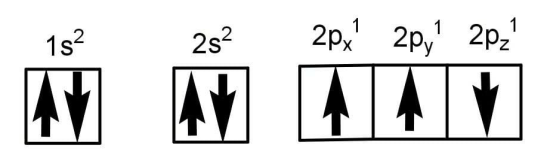
B.Total orbital angular momentum of an electron in ‘s’ orbital is equal to zero.
C.The value of m for is zero.
D.An orbital is designed by three quantum numbers while an electron in an atom is designed by four quantum numbers.

Answer
581.4k+ views
Hint: When only one electron is present in all the degenerate orbitals then they all must have the same spin. This is due to Hund’s rule of maximum multiplicity.
Complete step by step answer:
Let us look at each of the statements given in the options.
Nitrogen has atomic number 7 and the electronic configuration of nitrogen is:
\[1{{\text{s}}^2}2{{\text{s}}^2}2{{\text{p}}^3}\]
The electronic configuration is correctly represented by the orbital but the spin of electrons is incorrectly represented. According to Hund’s rule of maximum multiplicity the electrons exchange their position with each other, but if the spin of the electron is different in any one of the degenerate orbits then they cannot exchange the positions with each other and so the stability will decrease.
Degenerate orbits are those orbital which have the same energy such as the p orbital have the same energy as in case of nitrogen but the spin of one of them is opposite and hence is wrong. The correct electronic configuration of nitrogen will be:
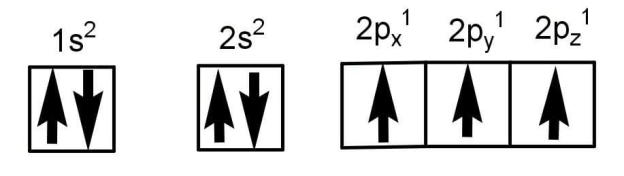
In this there is maximum exchange energy will be released and hence will be more stable.
The angular momentum for s orbital is zero because the value of l is zero. The value of the magnetic quantum number is zero for \[{{\text{d}}_{{{\text{z}}^2}}}\]. The orbits can be formed on the bases of values known for n, L and m quantum numbers, but for the position of electrons we also need to consider the spin quantum number and that makes four quantum numbers.
The correct option is A.
Note:
Hund’s rule states that for a given electronic configuration, the term with the maximum multiplicity has the lowest energy. The pairing in electrons only starts when each orbital is singly occupied.
Complete step by step answer:
Let us look at each of the statements given in the options.
Nitrogen has atomic number 7 and the electronic configuration of nitrogen is:
\[1{{\text{s}}^2}2{{\text{s}}^2}2{{\text{p}}^3}\]
The electronic configuration is correctly represented by the orbital but the spin of electrons is incorrectly represented. According to Hund’s rule of maximum multiplicity the electrons exchange their position with each other, but if the spin of the electron is different in any one of the degenerate orbits then they cannot exchange the positions with each other and so the stability will decrease.
Degenerate orbits are those orbital which have the same energy such as the p orbital have the same energy as in case of nitrogen but the spin of one of them is opposite and hence is wrong. The correct electronic configuration of nitrogen will be:

In this there is maximum exchange energy will be released and hence will be more stable.
The angular momentum for s orbital is zero because the value of l is zero. The value of the magnetic quantum number is zero for \[{{\text{d}}_{{{\text{z}}^2}}}\]. The orbits can be formed on the bases of values known for n, L and m quantum numbers, but for the position of electrons we also need to consider the spin quantum number and that makes four quantum numbers.
The correct option is A.
Note:
Hund’s rule states that for a given electronic configuration, the term with the maximum multiplicity has the lowest energy. The pairing in electrons only starts when each orbital is singly occupied.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




