
Which of the set of compounds do not have a conjugated system?
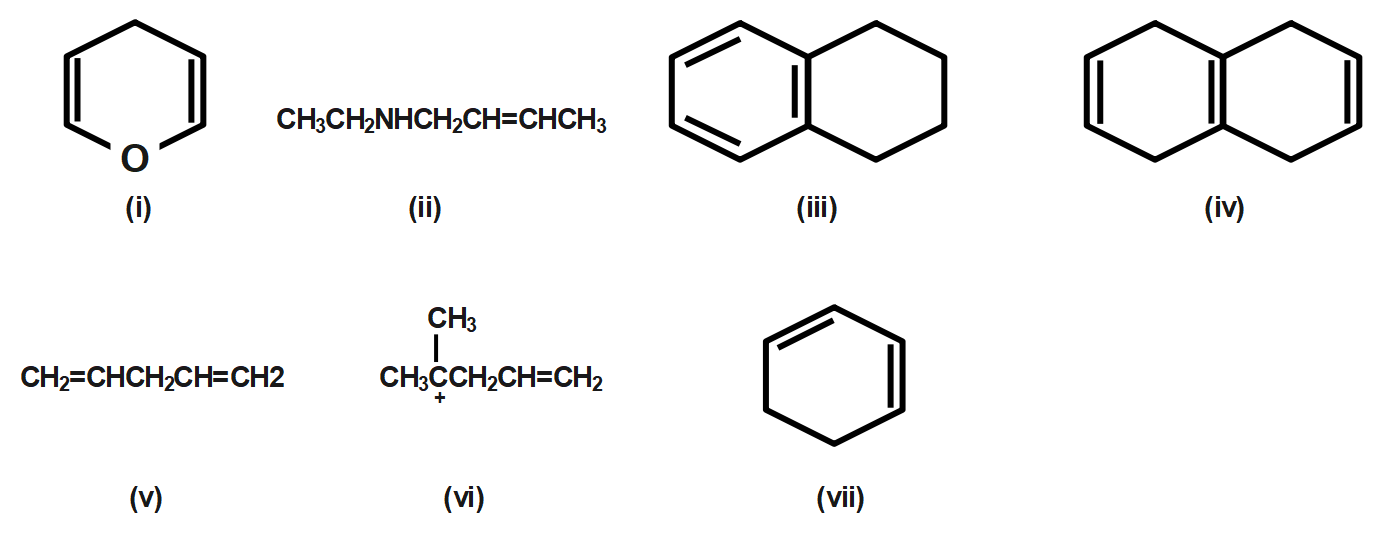
A. (ii), (iv), (v) and (vi)
B. (i), (ii), (v) and (vii)
C. (ii), (iii), (v) and (vi)
D. None of these.

Answer
535.5k+ views
Hint: Non aromatic or aromatic compounds can be checked by certain rules, if it follows then, aromatic and if not then, not aromatic. Aromaticity is a special property which is seen in organic compounds of ring-shaped, flat structures with a ring of conjugated systems that gives increased stability.
Complete step by step answer:
An aromatic compound contains a set of covalent bonds with specific characteristics
A delocalized conjugated system of electrons, which is an arrangement of alternating single and double bonds. It should be a coplanar structure having all the atoms in the same plane. It should follow Huckel’s rule, a number of delocalized electrons\[\left( 4n+2 \right)\], where \[n=0,1,2,3,\] and so on. Atoms should be arranged in one or more rings.
In a conjugated system, electrons are delocalized with the alternate single and double bond. Lone pairs can also be part of the system. Compounds (i),(iii) and (vii) have a conjugated system.
Therefore correct answer is option C that is three compounds which do not have a conjugated system are (ii),(iv),(v) and (vi)
So, the correct answer is Option C
Note: Note that the compound’s’ does not seem to be aromatic by its structure because the ring has $4$ electrons, which does not satisfy Huckel’s rule. But on resonance, with other atoms, a resonating structure is formed which is aromatic. So, do not judge Aromaticity by just looking at the compound, it can be aromatic in one form or another.
Complete step by step answer:
An aromatic compound contains a set of covalent bonds with specific characteristics
A delocalized conjugated system of electrons, which is an arrangement of alternating single and double bonds. It should be a coplanar structure having all the atoms in the same plane. It should follow Huckel’s rule, a number of delocalized electrons\[\left( 4n+2 \right)\], where \[n=0,1,2,3,\] and so on. Atoms should be arranged in one or more rings.
In a conjugated system, electrons are delocalized with the alternate single and double bond. Lone pairs can also be part of the system. Compounds (i),(iii) and (vii) have a conjugated system.
Therefore correct answer is option C that is three compounds which do not have a conjugated system are (ii),(iv),(v) and (vi)
So, the correct answer is Option C
Note: Note that the compound’s’ does not seem to be aromatic by its structure because the ring has $4$ electrons, which does not satisfy Huckel’s rule. But on resonance, with other atoms, a resonating structure is formed which is aromatic. So, do not judge Aromaticity by just looking at the compound, it can be aromatic in one form or another.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




