
Which of the given statements is true?
a.) The ionic end of soap dissolves in water while the carbon chain dissolves in oil
b.) The ionic end of soap dissolves in oil while the carbon chain dissolves in water
c.) The ionic end of soap and the carbon chain both dissolve in water
d.) The ionic end of soap dissolves in water while the carbon chain does not dissolve.
Answer
569.7k+ views
Hint: Soap: Soaps are sodium and potassium salts of long-chain fatty acids containing 12-18 carbon atoms. The general formula of soaps is $RCO{{O}^{-}}\text{ and N}{{\text{a}}^{+}}$ , where R = long-chain alkyl group.
Complete Solution :
- Soaps are prepared by a saponification reaction. Soaps are basically sodium or potassium salts of long-chain fatty acids such as palmitic acid, stearic acid, oleic acid, etc. in the industries the soaps are generally manufactured by heating fat with sodium hydroxide solution. Fats are nothing but triesters of glycerol and fatty acids.
- Glycerol is one of the organic compounds which contains around three carbon atoms and an OH group which are attached to each carbon atom, while fatty acids are long-chain carboxylic acids.
- We know that the soap molecules are present in water, the molecules arrange themselves as a cluster in which their hydrophobic ends are away from the water molecules, and their hydrophilic or also known as ionic ends are towards the water molecules. This whole process is known as micelle formation and the cluster formed by the soap molecules is called a micelle.
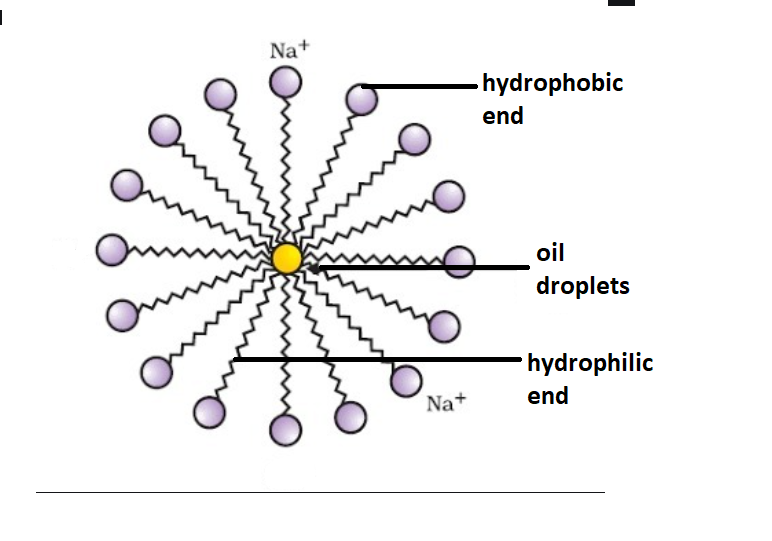
The polar head is hydrophilic while the nonpolar tail is hydrophobic. So the soap molecule in water is oriented with its head towards water and tail away from water.
As we know that like dissolves like so, the ionic end of soap dissolves in water while the carbon chain dissolves in oil.
So, the correct answer is “Option A”.
Note: The possibility to make a mistake is that you may choose option B. But an ionic end is a hydrophilic group (water-loving) and the alkyl chain is hydrophobic (water repellent).
Complete Solution :
- Soaps are prepared by a saponification reaction. Soaps are basically sodium or potassium salts of long-chain fatty acids such as palmitic acid, stearic acid, oleic acid, etc. in the industries the soaps are generally manufactured by heating fat with sodium hydroxide solution. Fats are nothing but triesters of glycerol and fatty acids.
- Glycerol is one of the organic compounds which contains around three carbon atoms and an OH group which are attached to each carbon atom, while fatty acids are long-chain carboxylic acids.
- We know that the soap molecules are present in water, the molecules arrange themselves as a cluster in which their hydrophobic ends are away from the water molecules, and their hydrophilic or also known as ionic ends are towards the water molecules. This whole process is known as micelle formation and the cluster formed by the soap molecules is called a micelle.

The polar head is hydrophilic while the nonpolar tail is hydrophobic. So the soap molecule in water is oriented with its head towards water and tail away from water.
As we know that like dissolves like so, the ionic end of soap dissolves in water while the carbon chain dissolves in oil.
So, the correct answer is “Option A”.
Note: The possibility to make a mistake is that you may choose option B. But an ionic end is a hydrophilic group (water-loving) and the alkyl chain is hydrophobic (water repellent).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




