
Which of the given statements for mercury cell are incorrect?
(i) Mercury cell is suitable for low current devices like hearing aids, watches, etc.
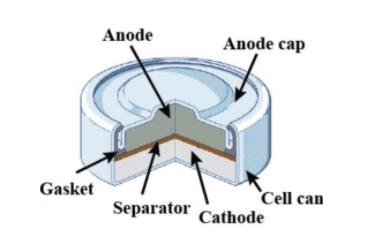
(ii) It consists of zinc-mercury amalgam as anode and a paste of HgO and carbon as the cathode.
(iii) The electrolyte is a paste of $Zn{{(OH)}_{2}}$ and $K{{O}_{2}}$.
(iv) The electrode reactions for the cell are
\[At\text{ }anode:\text{ }Zn(Hg)+{{H}_{2}}O\to Zn{{O}_{(s)}}+2O{{H}^{-}}+2{{e}^{-}}\]
\[At\text{ }cathode:\text{ }HgO+{{H}_{2}}O+2{{e}^{-}}\to H{{g}_{(l)}}+2O{{H}^{-}}\]
(A) (i) and (iii) only
(B) (i) and (ii) only
(C) (i), (iii) and (iv) only
(D) (iii) and (iv) only

Answer
573.6k+ views
Hint: The Mercury cell, also known as Mercury oxide battery or Mercury battery, is a primary cell, which is an electrochemical battery which is non-reusable and non-rechargeable. The zinc compound acts as an anode, where oxidation reaction takes place and mercury compound acts as a cathode where reduction reaction occurs in a mercury cell.
Complete answer:
- As we know, a mercury cell which is also called a mercury battery or mercuric oxide battery, is a non-rechargeable electrochemical battery and the electric cell produces current by irreversible chemical reactions.
- Mercury cell is an alkaline dry cell and has a low cell voltage around 1.34 V. Hence, mercury cell is used for low current devices such as button cells for watches, hearing aids, and calculators. Thus statement (i) is correct.
- In mercury cell, zinc-mercury amalgam ($Zn|ZnO$) is used as anode and mercuric oxide/Graphite ($Hg{{O}_{(s)}}|{{C}_{(s)}}$). Thus statement (ii) is also correct.
- In a mercury cell, potassium hydroxide or sodium hydroxide is used as an electrolyte and they ionize in a molten state to conduct electricity. Hence the electrolyte used is KOH not $K{{O}_{2}}$. Hence statement (iii) is incorrect.
- In Mercury cells, the cathode can be a mixture of mercuric oxide with manganese dioxide or HgO (pure mercury (II) oxide). Since MgO (magnesium oxide) is a non-conductor of electricity, some graphite are also mixed with this and the half-cell reaction at the cathode can be written as follows
\[HgO+{{H}_{2}}O+2{{e}^{-}}\to H{{g}_{(l)}}+O{{H}^{-}}\]
The half-cell reaction at anode can be written as follows
\[Zn(Hg)+2O{{H}^{-}}\to Zn{{O}_{(s)}}+{{H}_{2}}O+2{{e}^{-}}\]
This reaction is different from that is given in the statement (iv) and thus statement (iv) is also incorrect. From the above discussions it’s clear that options (iii) and (iv) are incorrect.
Therefore the answer is option (D) (iii) and (iv) only.
Note: It should be noted that, even though the mercury cells were highly popular during the 2nd World War, due to its economic factors and environmental risk factors such as inhalation of mercury vapor is harmful to the human body including organs like kidney, nervous system, digestive system, eye, skin and immunity systems , they have been replaced by other dry cells.
Complete answer:
- As we know, a mercury cell which is also called a mercury battery or mercuric oxide battery, is a non-rechargeable electrochemical battery and the electric cell produces current by irreversible chemical reactions.
- Mercury cell is an alkaline dry cell and has a low cell voltage around 1.34 V. Hence, mercury cell is used for low current devices such as button cells for watches, hearing aids, and calculators. Thus statement (i) is correct.
- In mercury cell, zinc-mercury amalgam ($Zn|ZnO$) is used as anode and mercuric oxide/Graphite ($Hg{{O}_{(s)}}|{{C}_{(s)}}$). Thus statement (ii) is also correct.
- In a mercury cell, potassium hydroxide or sodium hydroxide is used as an electrolyte and they ionize in a molten state to conduct electricity. Hence the electrolyte used is KOH not $K{{O}_{2}}$. Hence statement (iii) is incorrect.
- In Mercury cells, the cathode can be a mixture of mercuric oxide with manganese dioxide or HgO (pure mercury (II) oxide). Since MgO (magnesium oxide) is a non-conductor of electricity, some graphite are also mixed with this and the half-cell reaction at the cathode can be written as follows
\[HgO+{{H}_{2}}O+2{{e}^{-}}\to H{{g}_{(l)}}+O{{H}^{-}}\]
The half-cell reaction at anode can be written as follows
\[Zn(Hg)+2O{{H}^{-}}\to Zn{{O}_{(s)}}+{{H}_{2}}O+2{{e}^{-}}\]
This reaction is different from that is given in the statement (iv) and thus statement (iv) is also incorrect. From the above discussions it’s clear that options (iii) and (iv) are incorrect.
Therefore the answer is option (D) (iii) and (iv) only.
Note: It should be noted that, even though the mercury cells were highly popular during the 2nd World War, due to its economic factors and environmental risk factors such as inhalation of mercury vapor is harmful to the human body including organs like kidney, nervous system, digestive system, eye, skin and immunity systems , they have been replaced by other dry cells.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




