
Which of the following will not give iodoform test?
A.
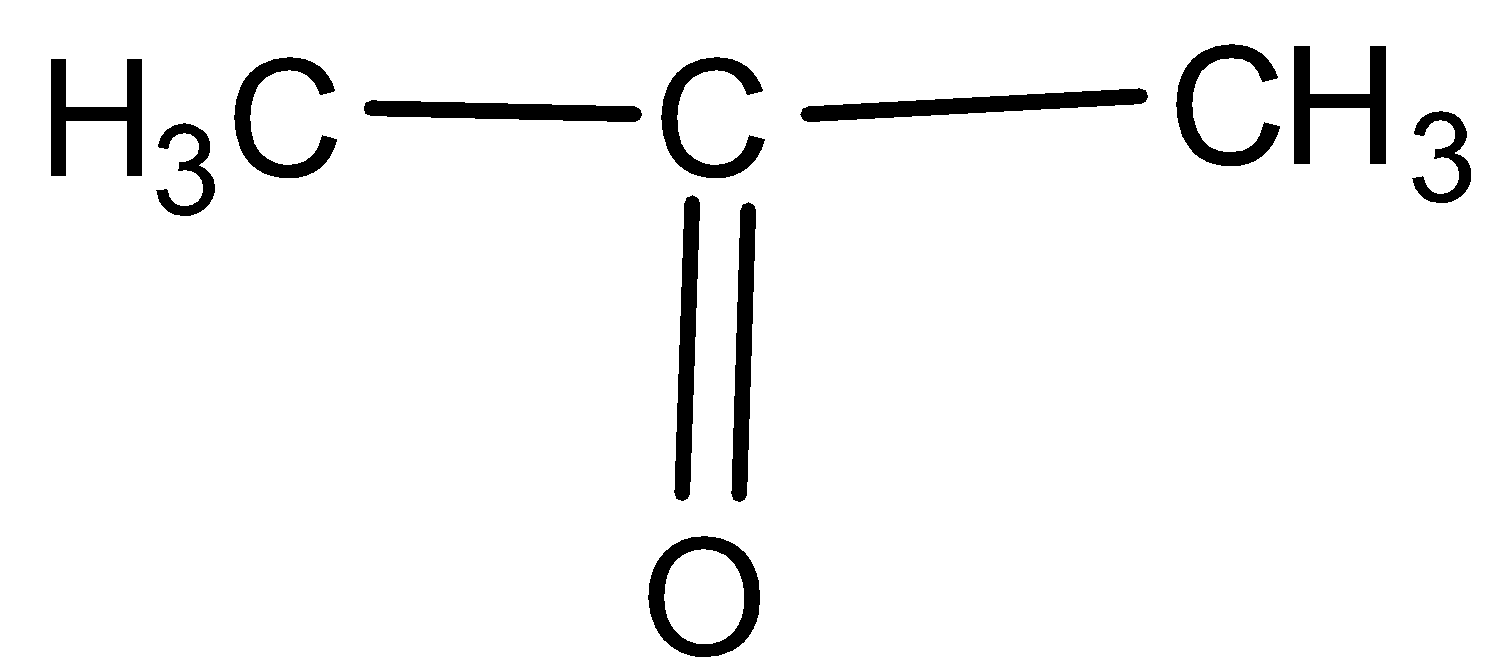
B.
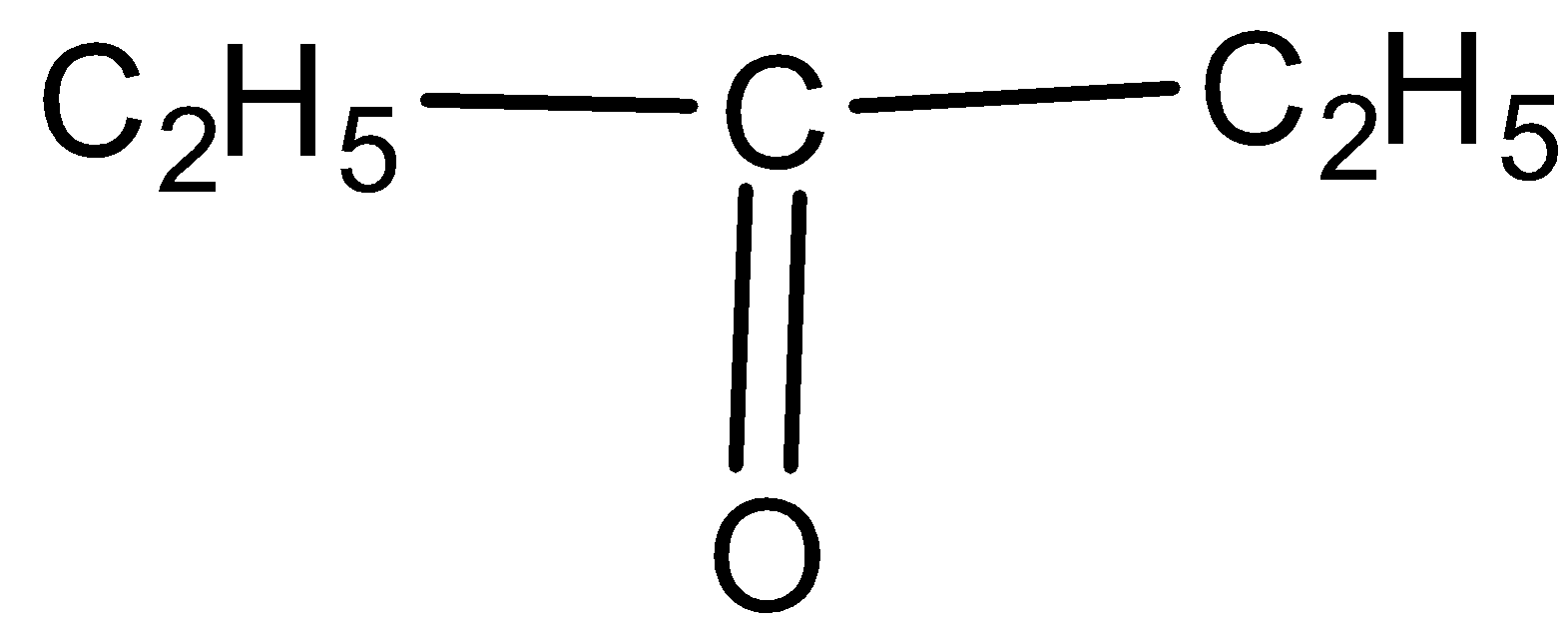
C.
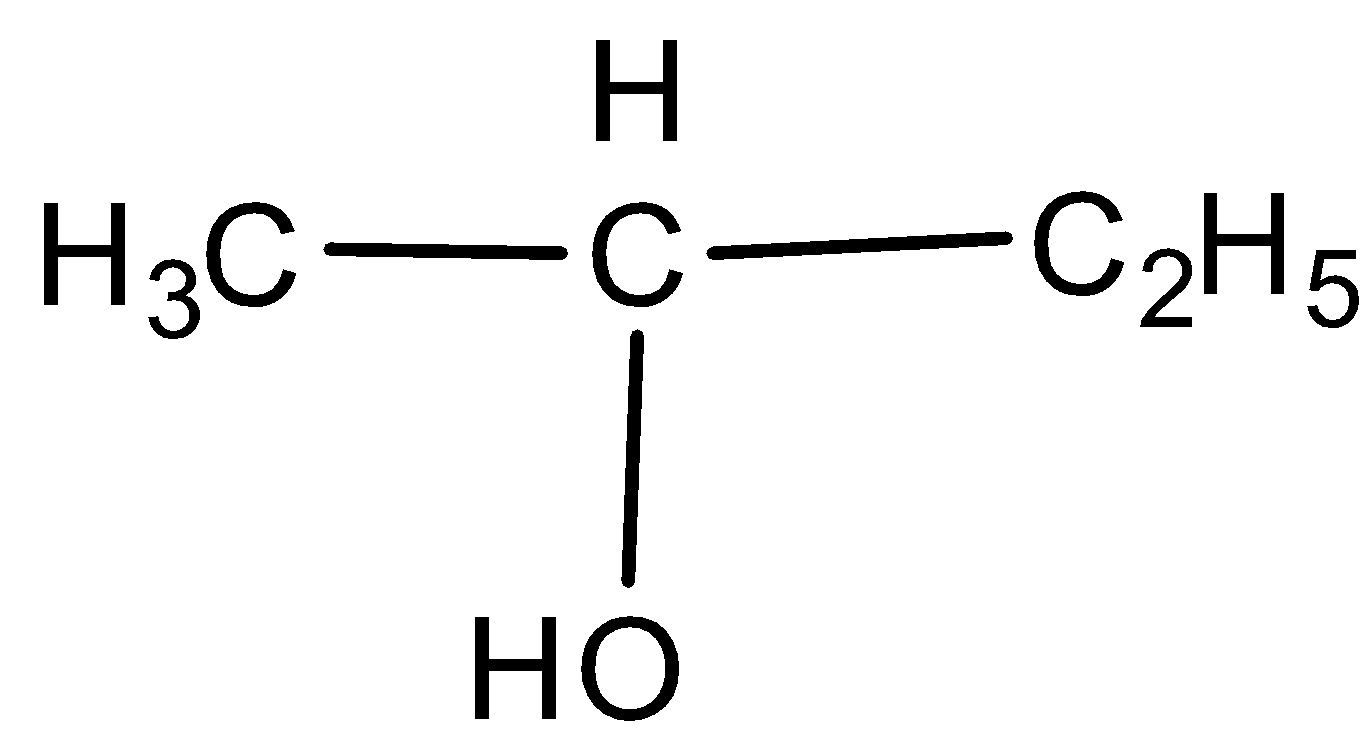
D.
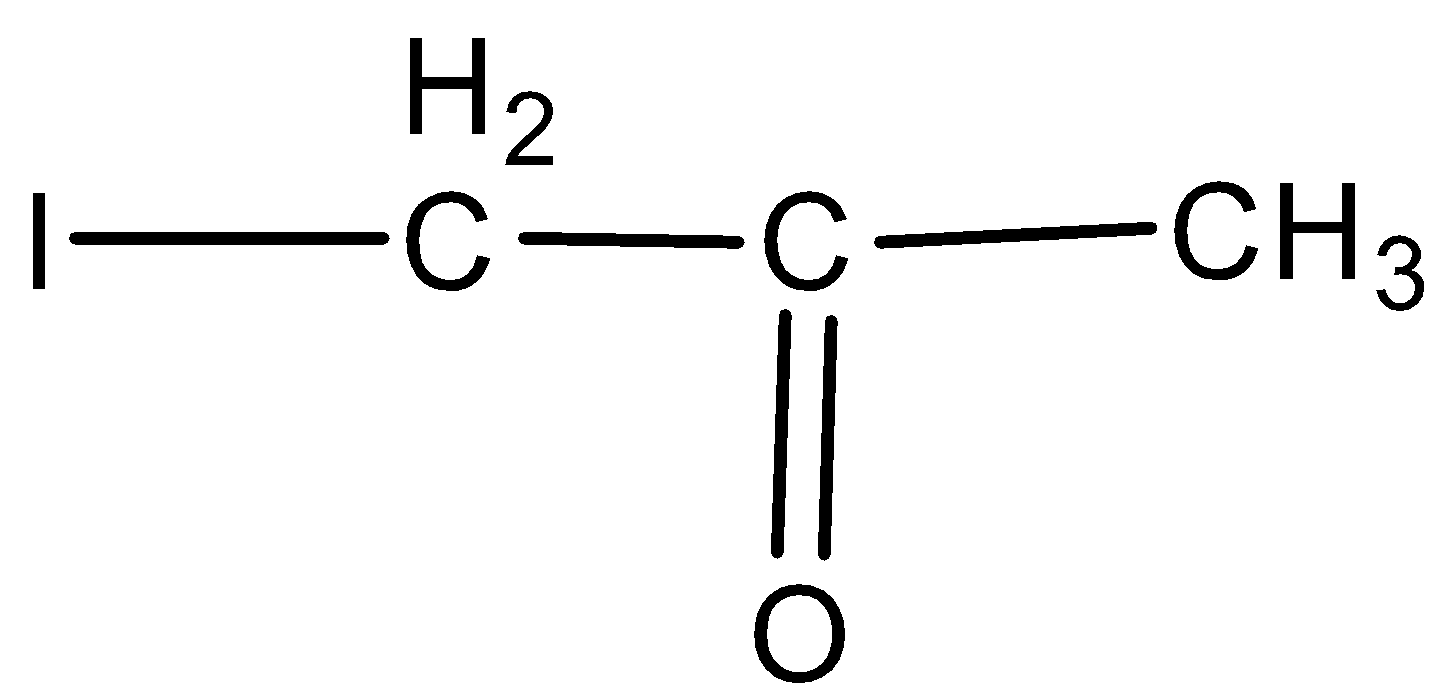




Answer
578.4k+ views
Hint: Iodoform test is given by all alcohols, acetaldehyde and all methyl ketones. The test is declared positive or negative by looking at the colour obtained by the precipitate after the completion of the reaction.
Complete answer:
-There are many functional groups that can attach themselves with the carbon chains to form compounds which show different characteristics and behavior compared to normal compounds.
-The functional groups are differentiated with one another through different reagents. They form different products on oxidation, reaction with 1 mole of grignard’s reagent, reaction with Tollen’s reagent, reaction of Benedict’s solution and many more reactions.
-Pure carbonyl compounds are aldehydes and ketones. Aldehyde gives black silver mirror with Tollen’s reagent $\left( AgN{{O}_{3}}+N{{H}_{4}}OH \right)$ and red precipitate with Fehling’s solution while ketone does not give this test. So they can be distinguished through these tests.
-Iodoform test is a test which can be given by alcohols also along with the methyl ketones and aldehyde. All alcohols give this test and form yellow precipitate after the completion of the reaction which tests for the presence of the functional groups.
-In the question, we are given alcohol, methyl ketone and ethyl ketone. All alcohols give positive iodoform test giving yellow precipitate but the iodoform test for ketones is selective. Only methyl ketones can give this test.
-As the test is positive only for the methyl ketones and not any other ketones, it is not given by ethyl ketones also. The ketones with methyl groups attached to any side of the carbon ring are methyl ketones and others are not methyl ketones.
-The reaction for alcohols and methyl ketones can be shown as
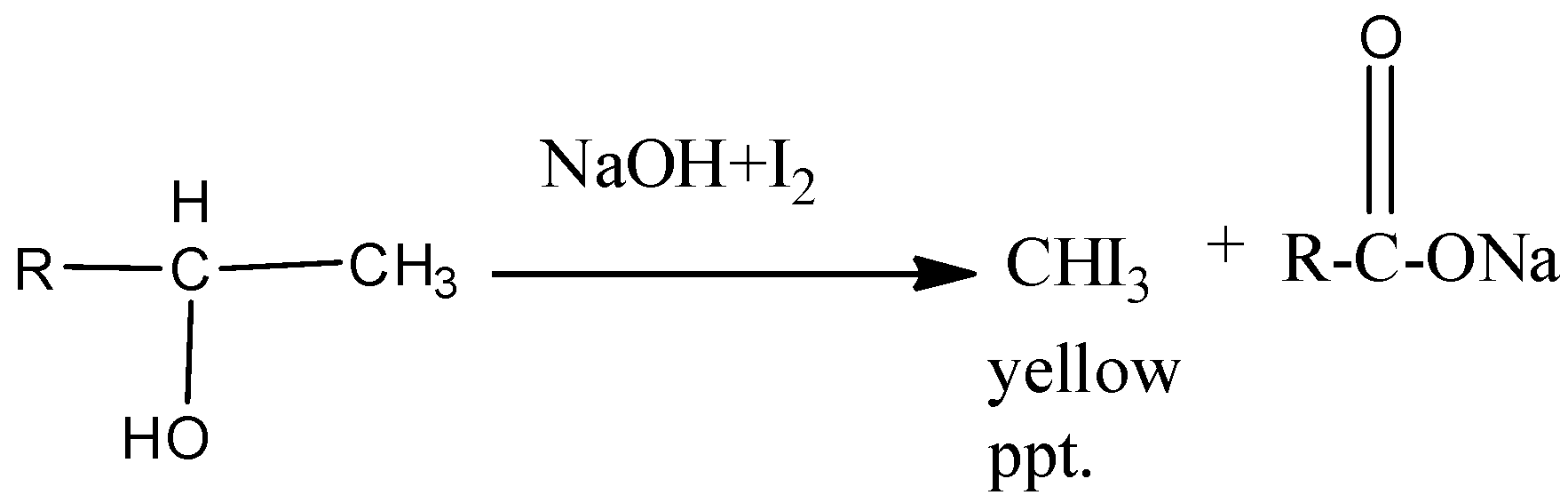
Therefore the correct option is B.
Note:
The iodoform test reaction occurs such that the product formed contains 1 less carbon atom as that carbon atom is the one which links to the iodine group leading to the formation of $CH{{I}_{3}}$ which verifies the iodoform test by giving the yellow precipitate.
Complete answer:
-There are many functional groups that can attach themselves with the carbon chains to form compounds which show different characteristics and behavior compared to normal compounds.
-The functional groups are differentiated with one another through different reagents. They form different products on oxidation, reaction with 1 mole of grignard’s reagent, reaction with Tollen’s reagent, reaction of Benedict’s solution and many more reactions.
-Pure carbonyl compounds are aldehydes and ketones. Aldehyde gives black silver mirror with Tollen’s reagent $\left( AgN{{O}_{3}}+N{{H}_{4}}OH \right)$ and red precipitate with Fehling’s solution while ketone does not give this test. So they can be distinguished through these tests.
-Iodoform test is a test which can be given by alcohols also along with the methyl ketones and aldehyde. All alcohols give this test and form yellow precipitate after the completion of the reaction which tests for the presence of the functional groups.
-In the question, we are given alcohol, methyl ketone and ethyl ketone. All alcohols give positive iodoform test giving yellow precipitate but the iodoform test for ketones is selective. Only methyl ketones can give this test.
-As the test is positive only for the methyl ketones and not any other ketones, it is not given by ethyl ketones also. The ketones with methyl groups attached to any side of the carbon ring are methyl ketones and others are not methyl ketones.
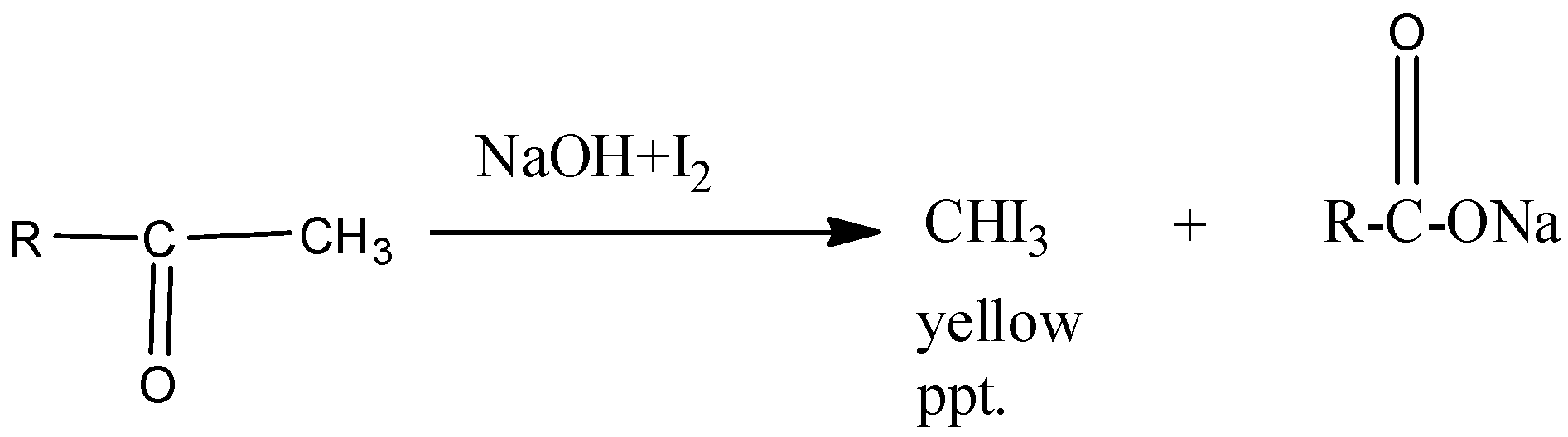
-The reaction for alcohols and methyl ketones can be shown as


Therefore the correct option is B.
Note:
The iodoform test reaction occurs such that the product formed contains 1 less carbon atom as that carbon atom is the one which links to the iodine group leading to the formation of $CH{{I}_{3}}$ which verifies the iodoform test by giving the yellow precipitate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




