
Which of the following will be readily soluble in water?
A)
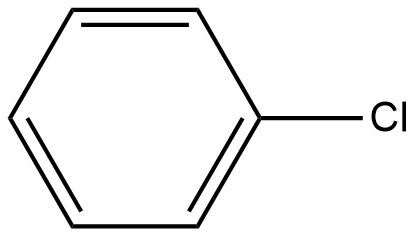
B)
C)
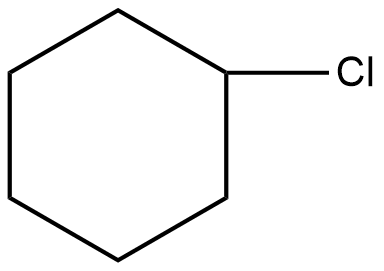
D)
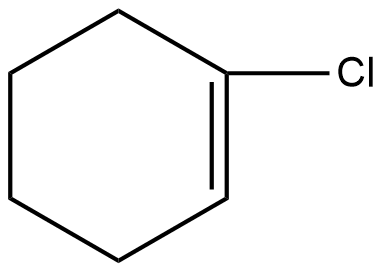




Answer
512.4k+ views
Hint: The reaction will occur through $SN_1$ mechanism as water is a weak nucleophile. The solubility of the compound will depend on the stability of the carbocation formed during the reaction.
Complete answer:
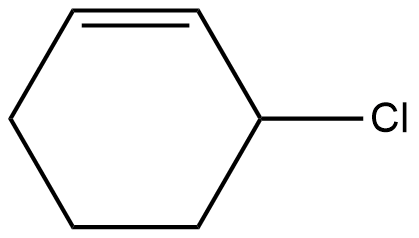
The compound given in the option B is 3-Chloro-1-cyclohexene.
The main reason behind this is the stability of carbocation formed during the $SN_1$ reaction.
Firstly, the substance in which we have to check the solubility is water, water is a weak nucleophile. Thus, the reaction will occur with $SN_1$ mechanism.
Nucleophile is basically a substance that has a tendency to donate electrons. $SN_1$ and $SN_2$ reactions are those reactions in which one nucleophile is substituted with another.
Now, as this reaction will be of $SN_1$ mechanism, it will be completed in two steps rather than completing in one step as $SN_2$ mechanism.
In those two steps, the first step is the formation of carbocation and the second step is the attack of nucleophile. Now, as said above, the better the stability of the carbocation, the more solubility it has.
The stability of carbocation also depends on two factors, resonance factor and inductive factor. Now, from all the above options, only option B has a resonance effect. Thus, the stability of the compound increases.
After the formation of carbocation, the nucleophile attacks the carbocation and forms 2-cyclohexen-1-ol. The image of 3-Chloro-1-cyclohexene and 2-cyclohexen-1-ol is shown below.
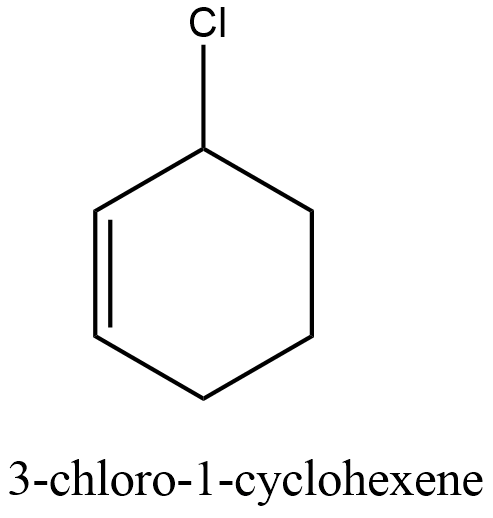
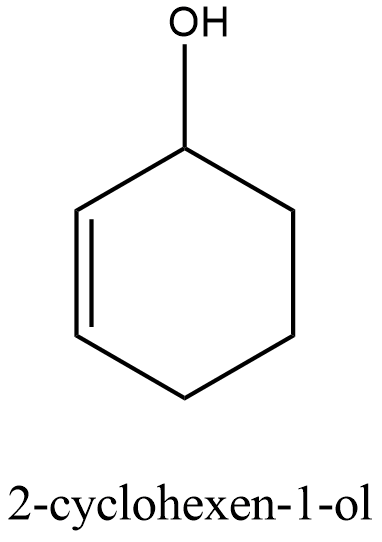
Therefore, the correct answer is option B.
Note:
The stability of the carbocation will depend upon two factors, resonance and inductivity. It is important to check every option with both of those factors.
Complete answer:
The compound given in the option B is 3-Chloro-1-cyclohexene.
The main reason behind this is the stability of carbocation formed during the $SN_1$ reaction.
Firstly, the substance in which we have to check the solubility is water, water is a weak nucleophile. Thus, the reaction will occur with $SN_1$ mechanism.
Nucleophile is basically a substance that has a tendency to donate electrons. $SN_1$ and $SN_2$ reactions are those reactions in which one nucleophile is substituted with another.
Now, as this reaction will be of $SN_1$ mechanism, it will be completed in two steps rather than completing in one step as $SN_2$ mechanism.
In those two steps, the first step is the formation of carbocation and the second step is the attack of nucleophile. Now, as said above, the better the stability of the carbocation, the more solubility it has.
The stability of carbocation also depends on two factors, resonance factor and inductive factor. Now, from all the above options, only option B has a resonance effect. Thus, the stability of the compound increases.
After the formation of carbocation, the nucleophile attacks the carbocation and forms 2-cyclohexen-1-ol. The image of 3-Chloro-1-cyclohexene and 2-cyclohexen-1-ol is shown below.


Therefore, the correct answer is option B.
Note:
The stability of the carbocation will depend upon two factors, resonance and inductivity. It is important to check every option with both of those factors.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




