
Which of the following statements is/are true for ${N_3}^ - $?
A) It has non-linear structure
B) It is called pseudohalogen
C) The formal oxidation state of nitrogen in this anions is $ - 1$
D) It is isoelectronic with $N{O_2}^ + $
Answer
560.7k+ views
Hint:Given compound ${N_3}^ - $ is known as an Azide ion. This ion has a total of twenty two electrons which means that each Nitrogen atom has seven electrons and the extra electron is denoted as the negative charge. The two nitrogen atoms form double bonds with the central nitrogen atom.
Complete step by step solution:
Given to us is an Azide ion. In this ion, two Nitrogen atoms form two double bonds with the central Nitrogen ion. Since the central atom is forming four bonds, it has a positive charge on it and since the other two nitrogen atoms form only two bonds, they have a negative change on them and hence the total charge is negative.
A) Let us draw the structure of the Azide ion with the above information.
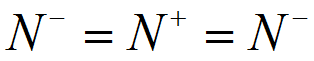
Hence, the structure is Linear.
B) Pseudohalogens or fake halogens are compounds that resemble the chemical properties of pure halogens. One of the examples of pseudohalogens is Azide ion.
C) The net charge on the Azide ion is $ - 1$ and hence the formal oxidation state of Nitrogen would be $ - 1$
D) In order to check whether Azide ion is isoelectronic with $N{O_2}^ + $, let us calculate the number of electrons present in it.
The number of electrons present in $N{O_2}^ + $ ion are $7 + 8 + 8 - 1 = 22$
We already know that the number of electrons present in ${N_3}^ - $ are $22$ and hence $N{O_2}^ + $ and ${N_3}^ - $ are isoelectronic.
So, clearly we can conclude that the correct answer is Option C.
Note:We know that the number of valence electrons in Nitrogen are $3$ and it is forming four bonds with two nitrogen atoms forming two bonds with each nitrogen atom. Hence its hybridization is sp and the shape is linear.
Complete step by step solution:
Given to us is an Azide ion. In this ion, two Nitrogen atoms form two double bonds with the central Nitrogen ion. Since the central atom is forming four bonds, it has a positive charge on it and since the other two nitrogen atoms form only two bonds, they have a negative change on them and hence the total charge is negative.
A) Let us draw the structure of the Azide ion with the above information.
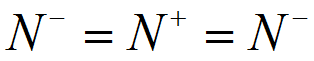
Hence, the structure is Linear.
B) Pseudohalogens or fake halogens are compounds that resemble the chemical properties of pure halogens. One of the examples of pseudohalogens is Azide ion.
C) The net charge on the Azide ion is $ - 1$ and hence the formal oxidation state of Nitrogen would be $ - 1$
D) In order to check whether Azide ion is isoelectronic with $N{O_2}^ + $, let us calculate the number of electrons present in it.
The number of electrons present in $N{O_2}^ + $ ion are $7 + 8 + 8 - 1 = 22$
We already know that the number of electrons present in ${N_3}^ - $ are $22$ and hence $N{O_2}^ + $ and ${N_3}^ - $ are isoelectronic.
So, clearly we can conclude that the correct answer is Option C.
Note:We know that the number of valence electrons in Nitrogen are $3$ and it is forming four bonds with two nitrogen atoms forming two bonds with each nitrogen atom. Hence its hybridization is sp and the shape is linear.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




