
Which of the following statements is not true about the hexagonal close packing?
(a)- The coordination number is 12
(b)- It has 74% packing efficiency
(c)- Tetrahedral voids of the second layer are covered by the spheres of the third layer.
(d)- In this arrangement the sphere of the fourth layer is exactly aligned with those of the first layer.
Answer
588.9k+ views
Hint: In hcp and ccp methods of stacking, a sphere is in contact with 6 other spheres in its layer. It also touches directly 3 spheres above and below. Thus, a sphere has 12 close neighbors. In hcp, the empty space is only 26%.
Complete step by step answer:
In hcp (hexagonal close packing), the third layer is placed over the second layer in such a way that the spheres cover the tetrahedral voids, a three-dimensional close packing is obtained where the spheres layers are in alternate pattern (i.e., the third layer is directly over the first layer and the fourth layer is over the second layer). Calling the first layer A and the second layer B, the arrangement is ABAB……..pattern or hexagonal close packing (hcp).
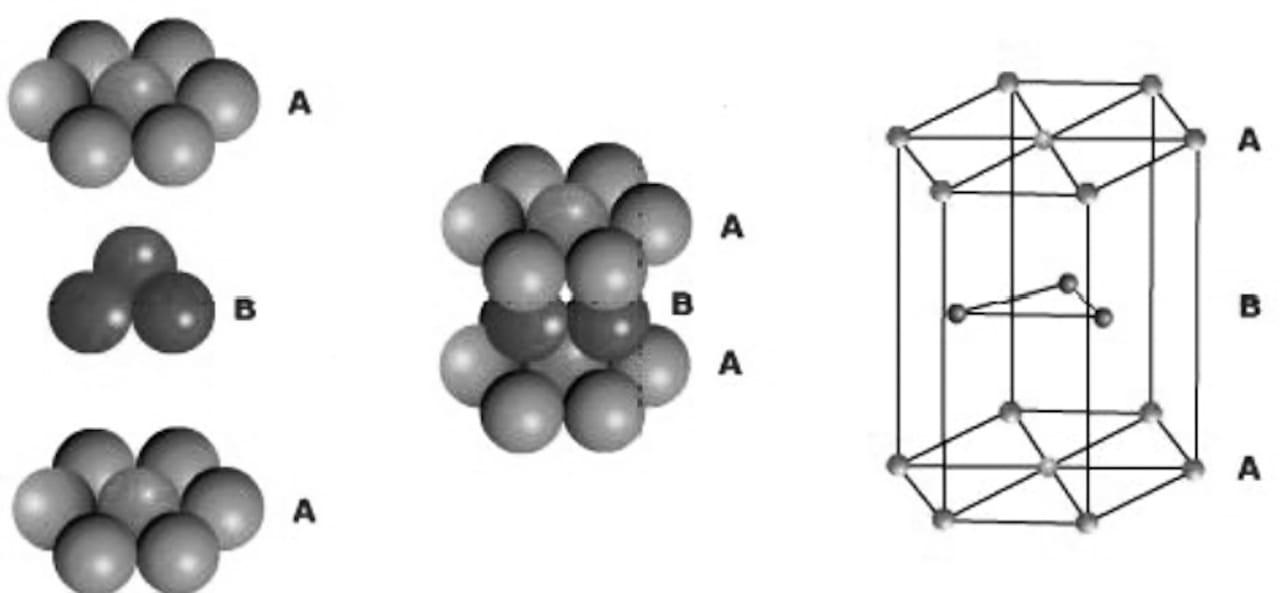
In hcp, the spheres occupy the maximum possible space and are about 74% of the available volume.
In hcp and ccp, one sphere is in contact with 6 spheres in its layer and 3 spheres in the above layer, and 3 spheres in the below layer. Thus, one sphere is surrounded by 12 spheres, so the coordination number is 12 in hcp.
So, in hcp
(a)- The coordination number is 12 is the correct statement.
(b)- It has 74% packing efficiency and is also correct because the sphere occupies 74% space.
(c)- Tetrahedral voids of the second layer are covered by the spheres of the third layer. This statement is also correct as discussed above.
(d)- In this arrangement the sphere of the fourth layer is exactly aligned with those of the first layer. This statement is incorrect, because they form ABAB patterns.
So, the correct answer is “Option D”.
Note: In the above discussion hcp refers to hexagonal close packing and ccp refers to cubic close packing. In ccp, the arrangement of the fourth layer is exactly aligned with those of the first layer which forms the ABCABC pattern. So, don't get confused between hcp and ccp structures.
Complete step by step answer:
In hcp (hexagonal close packing), the third layer is placed over the second layer in such a way that the spheres cover the tetrahedral voids, a three-dimensional close packing is obtained where the spheres layers are in alternate pattern (i.e., the third layer is directly over the first layer and the fourth layer is over the second layer). Calling the first layer A and the second layer B, the arrangement is ABAB……..pattern or hexagonal close packing (hcp).

In hcp, the spheres occupy the maximum possible space and are about 74% of the available volume.
In hcp and ccp, one sphere is in contact with 6 spheres in its layer and 3 spheres in the above layer, and 3 spheres in the below layer. Thus, one sphere is surrounded by 12 spheres, so the coordination number is 12 in hcp.
So, in hcp
(a)- The coordination number is 12 is the correct statement.
(b)- It has 74% packing efficiency and is also correct because the sphere occupies 74% space.
(c)- Tetrahedral voids of the second layer are covered by the spheres of the third layer. This statement is also correct as discussed above.
(d)- In this arrangement the sphere of the fourth layer is exactly aligned with those of the first layer. This statement is incorrect, because they form ABAB patterns.
So, the correct answer is “Option D”.
Note: In the above discussion hcp refers to hexagonal close packing and ccp refers to cubic close packing. In ccp, the arrangement of the fourth layer is exactly aligned with those of the first layer which forms the ABCABC pattern. So, don't get confused between hcp and ccp structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




