
Which of the following statements is not correct regarding diamond and graphite?
a.) In diamond, each carbon atom is covalently bonded to three other carbon atoms
b.) In graphite, each carbon atom is covalently bonded with three other carbon atoms in same plane
c.) The C - C bond length in graphite is intermediate between single and double bond distance length
d.) Diamond is layered structure ,the two layers joined by van der waal forces
Answer
598.8k+ views
Hint: Diamond is the crystalline structure in which there is no free electron while graphite is layered structure.
Complete step-by-step answer:
Structure of graphite:
Graphite is soft as well as a good conductor of electricity. It has a multilayered structure joined by van der waal forces.
In the graphite molecule, carbon atoms are covalently bonded three of its neighboring atoms in the same layer. So the fourth valence electron presents in between the different layers and is free to move around.
Structure of diamond:
Diamond is very hard and brittle. It is a crystalline solid which is covalently bonded.
In the diamond crystal, carbon atoms are covalently bonded to four of its neighboring atoms in the form of crystal. It is not layered structured. Their bonds are strong and unidirectional, therefore atoms are held strongly at their positions.
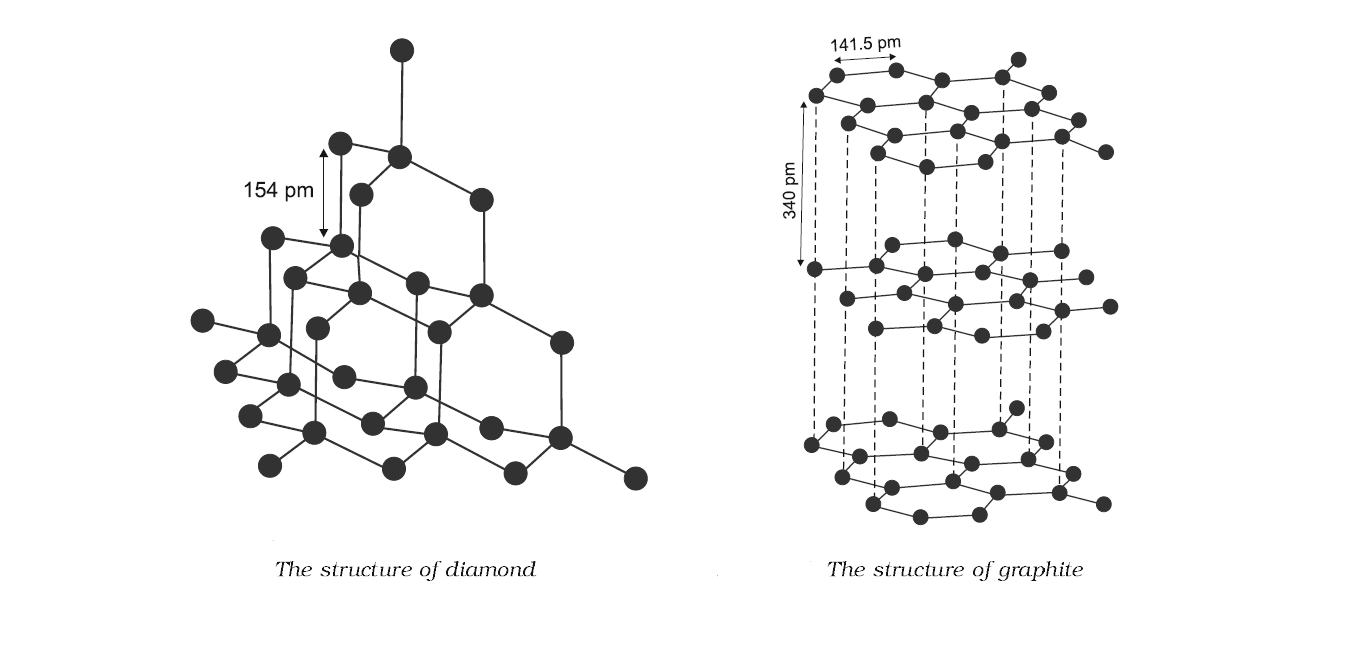
So, the statement A says that in diamond, each carbon atom is covalently bonded to three other carbon atoms. Yes it is true as shown in the above picture.
Statement B says in graphite, each carbon atom present in one layer is covalently bonded to three other in the same layer. Yes it is true graphite has a multilayered structure and layers are joined by weak van der waal forces.
Statement C says the C - C bond length in graphite is intermediate between single and double bond distance length. Yes it is true.
Statement D says Diamond is a layered structure and the two layers are joined by van der waal forces. This statement is false as it is not a layered structure it is a crystalline structure.
The correct option is D.
Note: Structure of diamond is crystalline. In diamond each atom present in diamond makes four other covalent bonds with neighboring carbons. Graphite is a layered structure and each layer one carbon atom bonds with 3 other carbon atoms while 1 electron remains free to move between layers.
Complete step-by-step answer:
Structure of graphite:
Graphite is soft as well as a good conductor of electricity. It has a multilayered structure joined by van der waal forces.
In the graphite molecule, carbon atoms are covalently bonded three of its neighboring atoms in the same layer. So the fourth valence electron presents in between the different layers and is free to move around.
Structure of diamond:
Diamond is very hard and brittle. It is a crystalline solid which is covalently bonded.
In the diamond crystal, carbon atoms are covalently bonded to four of its neighboring atoms in the form of crystal. It is not layered structured. Their bonds are strong and unidirectional, therefore atoms are held strongly at their positions.

So, the statement A says that in diamond, each carbon atom is covalently bonded to three other carbon atoms. Yes it is true as shown in the above picture.
Statement B says in graphite, each carbon atom present in one layer is covalently bonded to three other in the same layer. Yes it is true graphite has a multilayered structure and layers are joined by weak van der waal forces.
Statement C says the C - C bond length in graphite is intermediate between single and double bond distance length. Yes it is true.
Statement D says Diamond is a layered structure and the two layers are joined by van der waal forces. This statement is false as it is not a layered structure it is a crystalline structure.
The correct option is D.
Note: Structure of diamond is crystalline. In diamond each atom present in diamond makes four other covalent bonds with neighboring carbons. Graphite is a layered structure and each layer one carbon atom bonds with 3 other carbon atoms while 1 electron remains free to move between layers.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




