
Which of the following statements about $H_3PO_4$ is/are correct?
This question has multiple correct options
A.It is a strong tribasic acid
B.It is prepared by acidifying an aqueous solution of borax
C.It has a layer structure in which BO3 units are joined by hydrogen bond
D.It does not act as proton donor as it act as Lewis acid an accept the hydroxyl group
Answer
588k+ views
Hint: The boric acid is a weak acid which acts as an acid by forming $H_3O^+$ ion.Boric Acid is a monobasic Lewis acid with the chemical formula $H_3BO_3$. It is an acid-containing four atoms of oxygen, one atom of phosphorus, and three atoms of hydrogen. Boric acid is also known as acidum boricum, hydrogen borate, boracic acid, and orthoboric acid.
Complete step by step answer:
Let’s start by understanding the structure of $H_3BO_3$, The structure is given below:
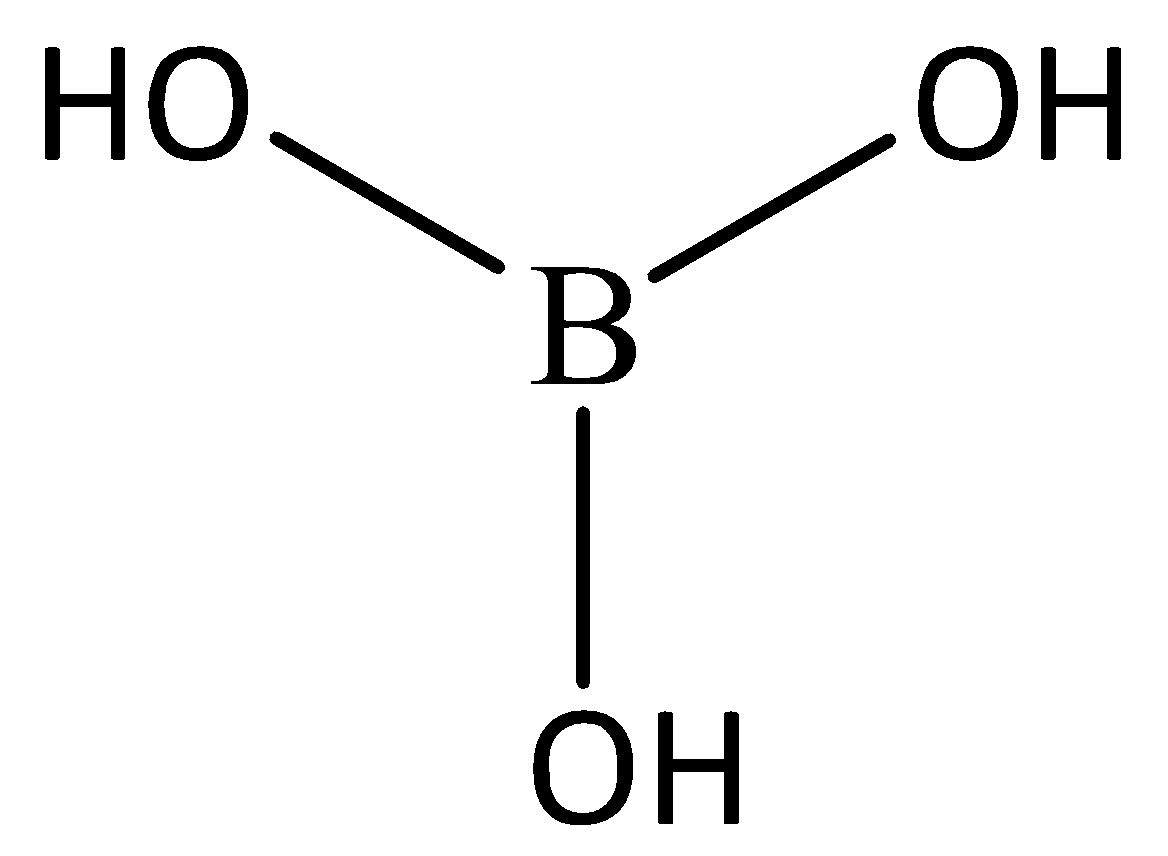
Looking at the structure we can determine that it is a planar structure, In this the crystalline structure is having the layers of $BO_3$ units which are joined together by hydrogen bonding. Hence, option C. is correct.
Now moving to check the option A, the $H_3BO_3$ is a weak monobasic acid, so clearly the option A is incorrect.
As for the preparation of $H_3BO_3$, in the preparation process of $H_3BO_3$ the borax is reacted with a mineral acid in aqueous medium. Borax is sodium tetraborate decahydrate and the reaction for the preparation of boric acid is as follows:
\[\;{\text{N}}{{\text{a}}_{\text{2}}}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{10}}{{\text{H}}_{\text{2}}}{\text{O + 2 HCl }} \to {\text{4 B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{[or }}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{] + 2 NaCl + 5 }}{{\text{H}}_{\text{2}}}{\text{O}}\]
Hence, the option C is also correct.
Coming to option D, The boric acid acts as a lewis acid by accepting the hydroxyl ion and forming H3O+ ions in the solution which results in an acidic medium.
\[{\text{B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}} \to {{\text{[B}}{\left( {{\text{OH}}} \right)_{\text{4}}}{\text{]}}^{\text{ - }}}{\text{ + H3}}{{\text{O}}^{{\text{ + }}}}\]
Hence, option D is also correct.
So, the correct answer to this question is options B, C, D.
Note:
Boric acid has a wide range of applications. The applications range from its use as an antiseptic, insecticide, flame retardant, neutron absorbent or precursor for other reactions. It exists in the form of a colourless crystal or in the form of white powder which is dissolved in water to get the acid.
Complete step by step answer:
Let’s start by understanding the structure of $H_3BO_3$, The structure is given below:

Looking at the structure we can determine that it is a planar structure, In this the crystalline structure is having the layers of $BO_3$ units which are joined together by hydrogen bonding. Hence, option C. is correct.
Now moving to check the option A, the $H_3BO_3$ is a weak monobasic acid, so clearly the option A is incorrect.
As for the preparation of $H_3BO_3$, in the preparation process of $H_3BO_3$ the borax is reacted with a mineral acid in aqueous medium. Borax is sodium tetraborate decahydrate and the reaction for the preparation of boric acid is as follows:
\[\;{\text{N}}{{\text{a}}_{\text{2}}}{{\text{B}}_{\text{4}}}{{\text{O}}_{\text{7}}}{\text{10}}{{\text{H}}_{\text{2}}}{\text{O + 2 HCl }} \to {\text{4 B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{[or }}{{\text{H}}_{\text{3}}}{\text{B}}{{\text{O}}_{\text{3}}}{\text{] + 2 NaCl + 5 }}{{\text{H}}_{\text{2}}}{\text{O}}\]
Hence, the option C is also correct.
Coming to option D, The boric acid acts as a lewis acid by accepting the hydroxyl ion and forming H3O+ ions in the solution which results in an acidic medium.
\[{\text{B}}{\left( {{\text{OH}}} \right)_{\text{3}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}} \to {{\text{[B}}{\left( {{\text{OH}}} \right)_{\text{4}}}{\text{]}}^{\text{ - }}}{\text{ + H3}}{{\text{O}}^{{\text{ + }}}}\]
Hence, option D is also correct.
So, the correct answer to this question is options B, C, D.
Note:
Boric acid has a wide range of applications. The applications range from its use as an antiseptic, insecticide, flame retardant, neutron absorbent or precursor for other reactions. It exists in the form of a colourless crystal or in the form of white powder which is dissolved in water to get the acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




