
Which of the following species have undistorted octahedral structures?
1. \[S{F_6}\]
2. \[PF_6^ - \]
3. \[SiF_6^{2 - }\]
4. \[Xe{F_6}\]
A. 2,3 and 4
B. 1,3 and 4
C. 2 and 3
D. 1, 2 and 3
Answer
564.6k+ views
Hint: In the undistorted octahedral shape of the molecule the central atom does not possess any lone pairs as the valence electron is used in forming six chemical bonds with six fluorine atoms.
Complete step by step answer:
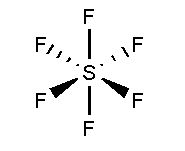
In \[S{F_6}\], the Sulphur atom is surrounded by six fluorine atoms. The atomic number of Sulphur is 16 and the electronic configuration is \[[Ne]3{s^2}3{p^4}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 12 valence electrons form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It is arranged in undistorted octahedral shape.
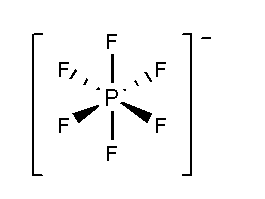
In \[PF_6^ - \], the phosphorus atom is surrounded by six fluorine atoms. The atomic number of phosphorus is 15 and the electronic configuration is \[[Ne]3{s^2}3{p^3}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 11 valence electrons and one negative charge form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. According to VSEPR theory \[PF_6^ - \]show undistorted octahedral structures
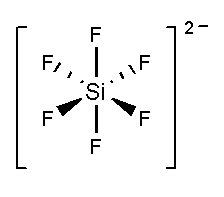
In \[SiF_6^{2 - }\], the silicon atom is surrounded by six fluorine atoms. The atomic number of silicon is 14 and the electronic configuration is \[[Ne]3{s^2}3{p^2}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 10 valence electrons and two negative charges form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It forms an undistorted octahedral shape.
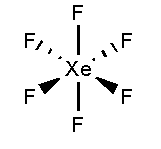
In \[Xe{F_6}\], the xenon atom is surrounded by six fluorine atoms. The atomic number of xenon is 54 and the electronic configuration is \[[Kr]4{d^{10}}5{s^2}5{p^6}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 12 valence electrons form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It forms an octahedral shape.
Therefore, the correct option is D
Note:
In \[Xe{F_6}\] as the valence electrons in xenon is 8 out of which six forms bond and the remaining two electrons and present as lone pairs and located in the \[s{p^3}{d^3}\]hybrid orbital. Therefore it forms a distorted octahedral complex.
Complete step by step answer:

In \[S{F_6}\], the Sulphur atom is surrounded by six fluorine atoms. The atomic number of Sulphur is 16 and the electronic configuration is \[[Ne]3{s^2}3{p^4}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 12 valence electrons form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It is arranged in undistorted octahedral shape.

In \[PF_6^ - \], the phosphorus atom is surrounded by six fluorine atoms. The atomic number of phosphorus is 15 and the electronic configuration is \[[Ne]3{s^2}3{p^3}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 11 valence electrons and one negative charge form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. According to VSEPR theory \[PF_6^ - \]show undistorted octahedral structures

In \[SiF_6^{2 - }\], the silicon atom is surrounded by six fluorine atoms. The atomic number of silicon is 14 and the electronic configuration is \[[Ne]3{s^2}3{p^2}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 10 valence electrons and two negative charges form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It forms an undistorted octahedral shape.

In \[Xe{F_6}\], the xenon atom is surrounded by six fluorine atoms. The atomic number of xenon is 54 and the electronic configuration is \[[Kr]4{d^{10}}5{s^2}5{p^6}\]. The atomic number of fluorine is 9 and the electronic configuration of \[[He]2{s^2}2{p^5}\]. Total 12 valence electrons form six bonds with six fluorine atoms and three lone pairs present in each fluorine atom. It forms an octahedral shape.
Therefore, the correct option is D
Note:
In \[Xe{F_6}\] as the valence electrons in xenon is 8 out of which six forms bond and the remaining two electrons and present as lone pairs and located in the \[s{p^3}{d^3}\]hybrid orbital. Therefore it forms a distorted octahedral complex.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




