
Which of the following sawhorse representations is correct for the given Newman projection.

A.
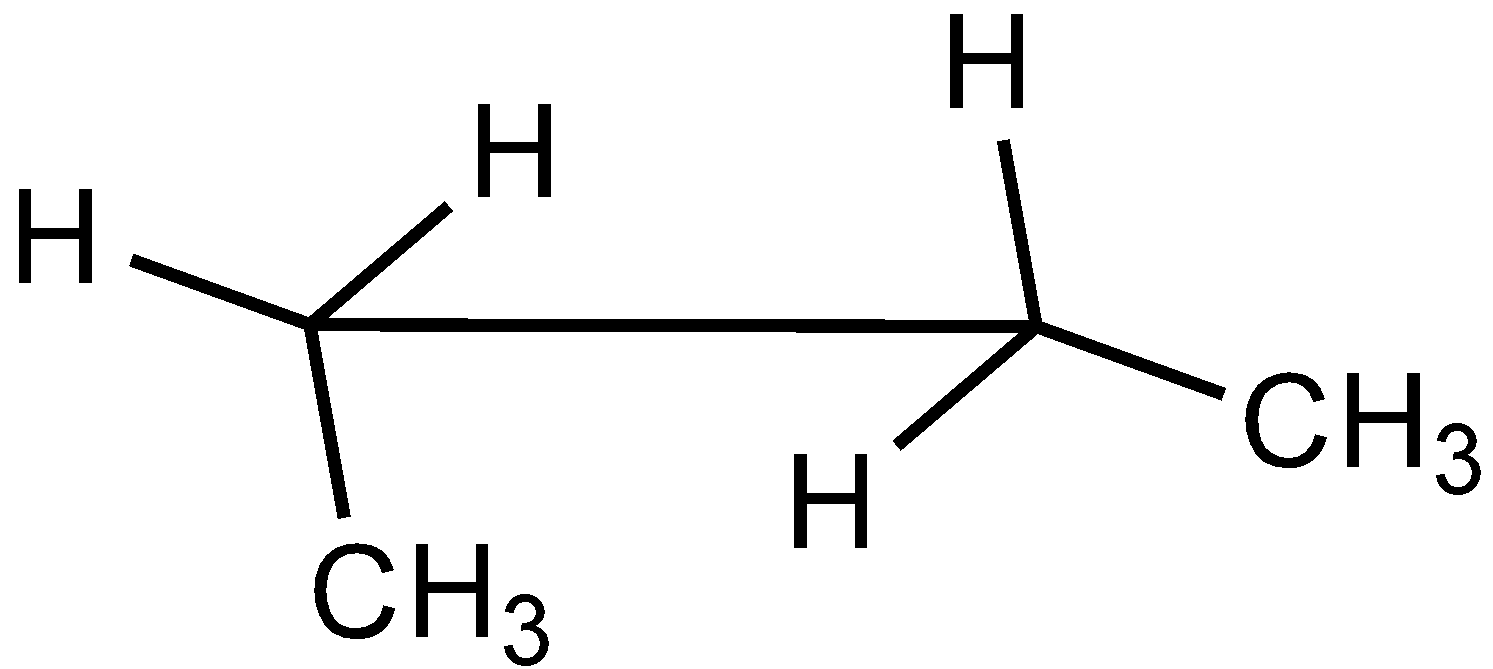
B.
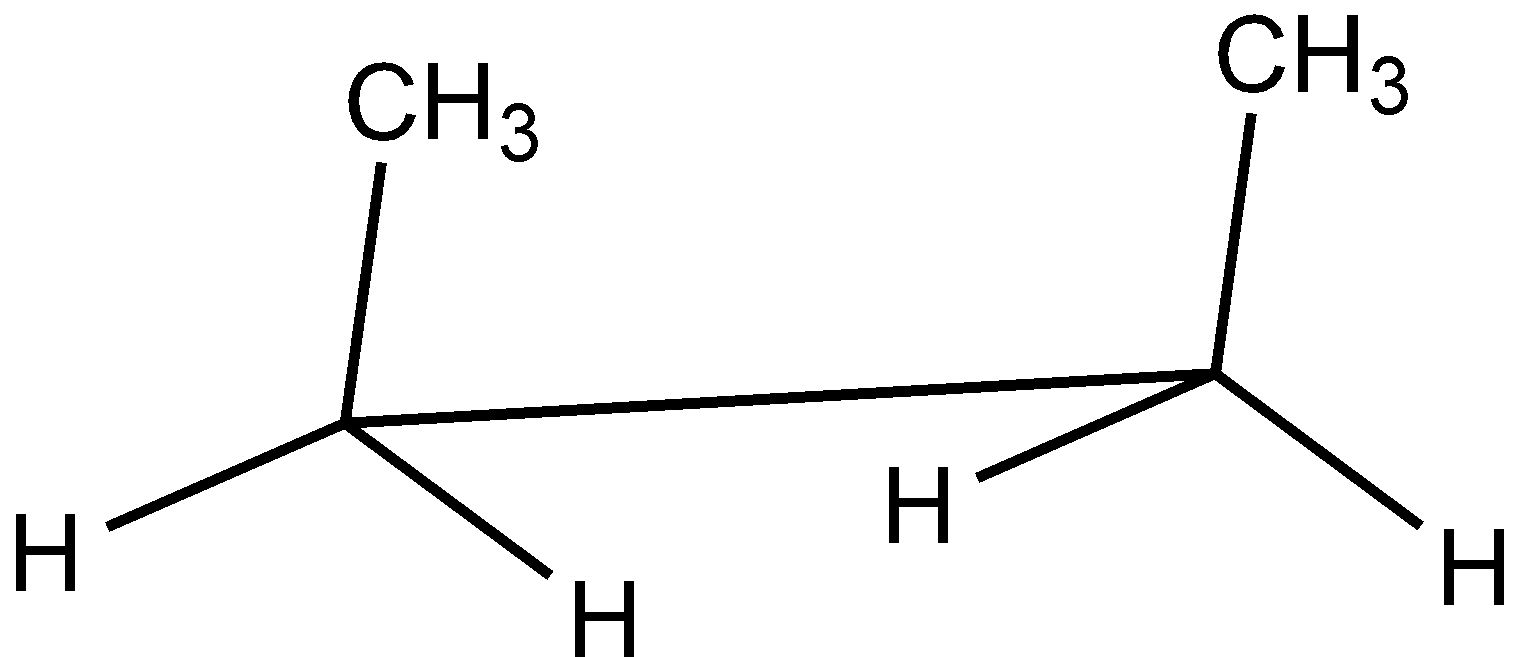
C.
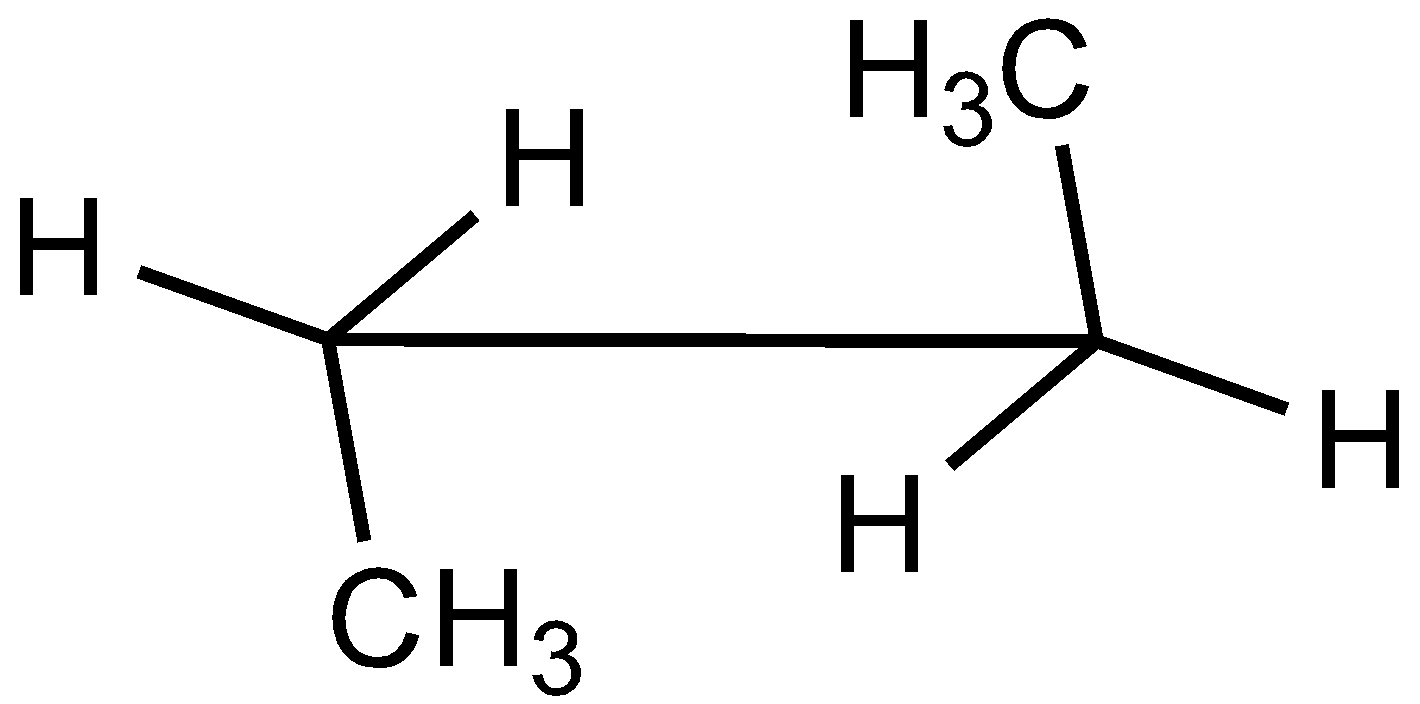
D.
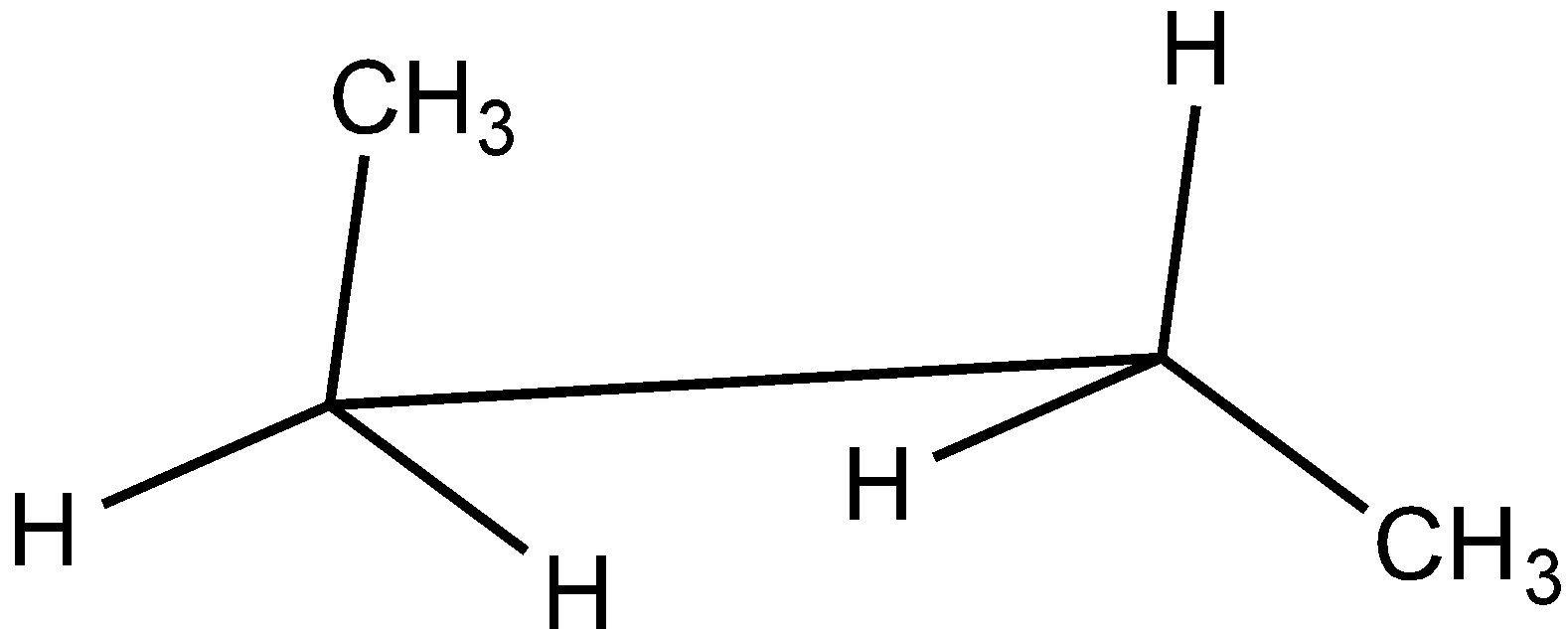





Answer
572.1k+ views
Hint: As we know that certain molecules have the property of isomerism and the different arrangements of the compounds arising due to sigma bond rotation are known as conformations and they tend to have conformational isomerism. One drawing convention that helps visualize these structures better is known as Newman projection.
Complete step by step answer:
In the case of Newman projection, the carbon-carbon bond is compressed whereas they are properly visible in Sawhorse projection. In the case of sawhorse projection, the molecule is drawn showing a carbon-carbon bond, and the groups connected between front and back carbon are drawn and the angle between the sticks is $120^\circ $. As per the question we are given the Newman projection for Butane in staggered conformation.
So the correct seahorse representation for the given Newman projection of Butane is:
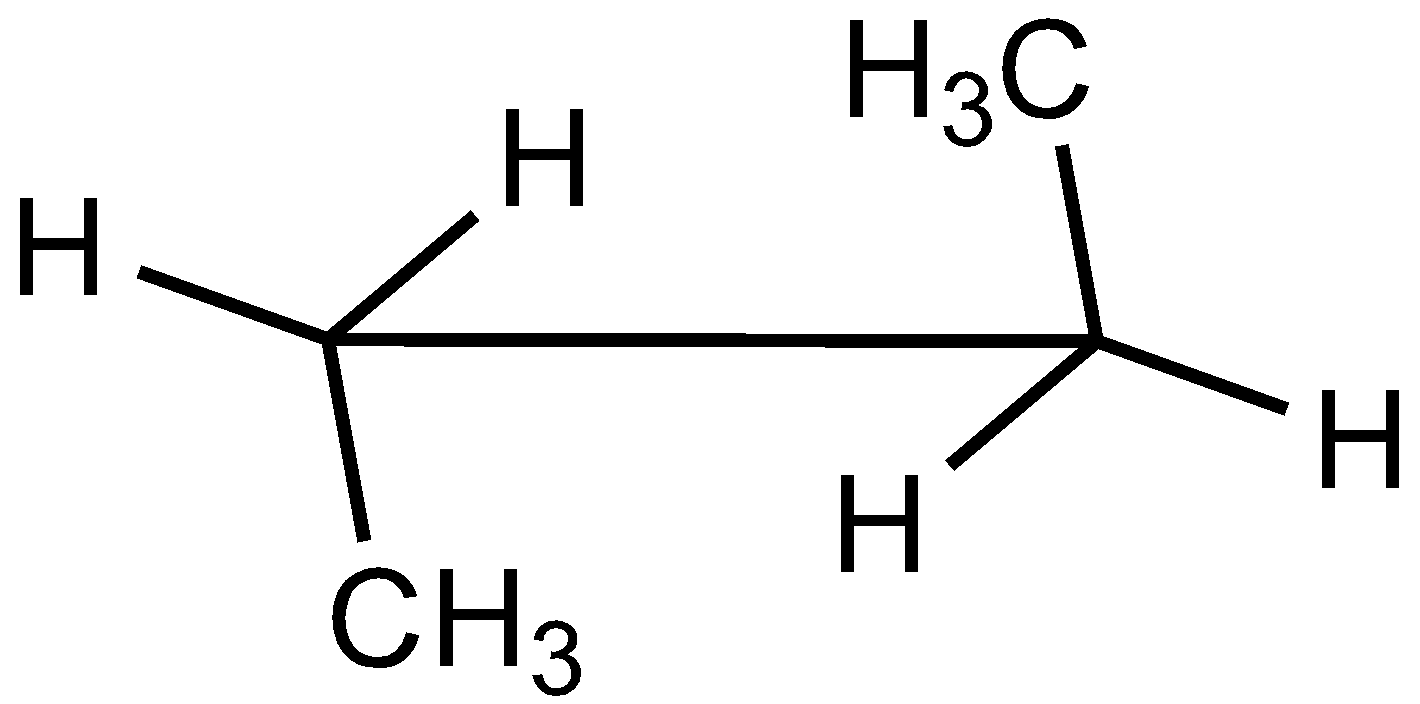
So, the correct answer is Option C.
Additional Information:
To determine the 3D shapes of the molecules on a 2D page in an unambiguous manner we prepare different types of diagrams that are:
1.Wedge Hash: These are the most common representations and are used to show the 3D shape and sp3 hybridized.
2.Sawhorse diagram: They are the same as the wedge hash diagram but don't show the perspective the way it is shown in the wedge-hash diagram.
3.Newman Projections: In this diagram, we look to the central atom and draw other atoms as to how they would be around it.
4.Fischer Projection: They are mostly used in representing biomolecules and are best when we need to represent multiple stereocenters.
Note: Both Newman and seahorse projections are used to represent eclipsed and staggered conformations of Conformational isomers. Newman projections are mostly used in stereochemistry for Alkanes, whereas seahorse projections help us determine if the two molecules are enantiomers or diastereomers. Also using seahorse projection, it is easier to understand if the structures are superimposable.
Complete step by step answer:
In the case of Newman projection, the carbon-carbon bond is compressed whereas they are properly visible in Sawhorse projection. In the case of sawhorse projection, the molecule is drawn showing a carbon-carbon bond, and the groups connected between front and back carbon are drawn and the angle between the sticks is $120^\circ $. As per the question we are given the Newman projection for Butane in staggered conformation.
So the correct seahorse representation for the given Newman projection of Butane is:

So, the correct answer is Option C.
Additional Information:
To determine the 3D shapes of the molecules on a 2D page in an unambiguous manner we prepare different types of diagrams that are:
1.Wedge Hash: These are the most common representations and are used to show the 3D shape and sp3 hybridized.
2.Sawhorse diagram: They are the same as the wedge hash diagram but don't show the perspective the way it is shown in the wedge-hash diagram.
3.Newman Projections: In this diagram, we look to the central atom and draw other atoms as to how they would be around it.
4.Fischer Projection: They are mostly used in representing biomolecules and are best when we need to represent multiple stereocenters.
Note: Both Newman and seahorse projections are used to represent eclipsed and staggered conformations of Conformational isomers. Newman projections are mostly used in stereochemistry for Alkanes, whereas seahorse projections help us determine if the two molecules are enantiomers or diastereomers. Also using seahorse projection, it is easier to understand if the structures are superimposable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




