
Which of the following ring compounds obeys Huckel’s rule?
(A)- ${{C}_{4}}H_{4}^{-1}$
(B)- ${{C}_{4}}H_{4}^{+1}$
(C)- ${{C}_{4}}H_{4}^{-2}$
(D)- ${{C}_{4}}{{H}_{4}}$
Answer
586.2k+ views
Hint: Erich Huckel in 1931 proposed a condition for a compound to be aromatic popularly known as Huckel’s rule. It states that a cyclic, planar and conjugated system having (4n+2)$\pi $ electrons, is considered to be aromatic.
Complete answer:
Aromatic compounds must obey Huckel’s rule and contain a certain number of $\pi $ electrons which should be equal to 4n+2. Here, n can be zero or any other positive integer. Therefore, compounds containing$2\pi $, $6\pi $, $10\pi $, $14\pi $ and so on electrons are aromatic.
Let us try to find the ring compound which obeys Huckel’s rule form the above given options.
${{C}_{4}}H_{4}^{-1}$
Possible ring structures with ${{C}_{4}}H_{4}^{-1}$ are shown below:
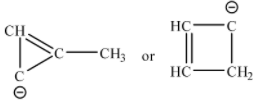
Neither of the compounds follow Huckel’s rule. In the three-membered ring compound the total number of $\pi $ electrons is 4, which is not a multiple of (4n+2)$\pi $. Ring compounds containing $4\pi $ electrons are anti-aromatic in nature. In the four-membered ring, there is a $s{{p}^{3}}$ hybridized tetrahedral carbon, so the ring is no longer planar. Thus, Huckel’s rule cannot be applied.
${{C}_{4}}H_{4}^{+1}$
Possible structure for ${{C}_{4}}H_{4}^{+1}$ is:
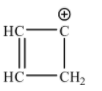
This ring is not planar as it contains a $s{{p}^{3}}$ hybridized carbon. Therefore, Huckel’s rule is not applicable.
${{C}_{4}}H_{4}^{-2}$
With the given molecular formula the possible ring structure is shown below:
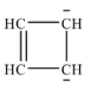
This ring compound contains $6\pi $ electrons which is equal to (4n+2)$\pi $ when n=1. Therefore, it obeys Huckel’s rule and is aromatic.
${{C}_{4}}{{H}_{4}}$
Structure of ${{C}_{4}}{{H}_{4}}$ is given as:
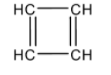
This cyclic compound contains $4\pi $ electrons and thus, it is antiaromatic. This compound also does not follow Huckel’s rule.
The only compound following Huckel’s rule is ${{C}_{4}}H_{4}^{-2}$.
Hence, the correct option is (C).
Additional information:
Compounds which lacks one or more of the four requirements, i.e. cyclic, planar, conjugated and (4n+2)$\pi $ electrons for aromatic; 4n$\pi $ for antiaromatic, to be aromatic or antiaromatic are considered as non aromatic.
Stability of the compounds follows the following order:
Aromatic > Non-aromatic> Anti-aromatic
Note:
Carefully count the number of $\pi $ electrons in each compound. One lone pair of electrons is counted as 2$\pi $ electrons and one double bond or triple bond in a ring also contributes 2$\pi $ electrons.
Complete answer:
Aromatic compounds must obey Huckel’s rule and contain a certain number of $\pi $ electrons which should be equal to 4n+2. Here, n can be zero or any other positive integer. Therefore, compounds containing$2\pi $, $6\pi $, $10\pi $, $14\pi $ and so on electrons are aromatic.
Let us try to find the ring compound which obeys Huckel’s rule form the above given options.
${{C}_{4}}H_{4}^{-1}$
Possible ring structures with ${{C}_{4}}H_{4}^{-1}$ are shown below:
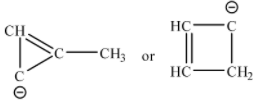
Neither of the compounds follow Huckel’s rule. In the three-membered ring compound the total number of $\pi $ electrons is 4, which is not a multiple of (4n+2)$\pi $. Ring compounds containing $4\pi $ electrons are anti-aromatic in nature. In the four-membered ring, there is a $s{{p}^{3}}$ hybridized tetrahedral carbon, so the ring is no longer planar. Thus, Huckel’s rule cannot be applied.
${{C}_{4}}H_{4}^{+1}$
Possible structure for ${{C}_{4}}H_{4}^{+1}$ is:

This ring is not planar as it contains a $s{{p}^{3}}$ hybridized carbon. Therefore, Huckel’s rule is not applicable.
${{C}_{4}}H_{4}^{-2}$
With the given molecular formula the possible ring structure is shown below:

This ring compound contains $6\pi $ electrons which is equal to (4n+2)$\pi $ when n=1. Therefore, it obeys Huckel’s rule and is aromatic.
${{C}_{4}}{{H}_{4}}$
Structure of ${{C}_{4}}{{H}_{4}}$ is given as:

This cyclic compound contains $4\pi $ electrons and thus, it is antiaromatic. This compound also does not follow Huckel’s rule.
The only compound following Huckel’s rule is ${{C}_{4}}H_{4}^{-2}$.
Hence, the correct option is (C).
Additional information:
Compounds which lacks one or more of the four requirements, i.e. cyclic, planar, conjugated and (4n+2)$\pi $ electrons for aromatic; 4n$\pi $ for antiaromatic, to be aromatic or antiaromatic are considered as non aromatic.
Stability of the compounds follows the following order:
Aromatic > Non-aromatic> Anti-aromatic
Note:
Carefully count the number of $\pi $ electrons in each compound. One lone pair of electrons is counted as 2$\pi $ electrons and one double bond or triple bond in a ring also contributes 2$\pi $ electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




