
Which of the following potential energy diagrams represents the ${{S}_{N}}1$ reaction?
(A)
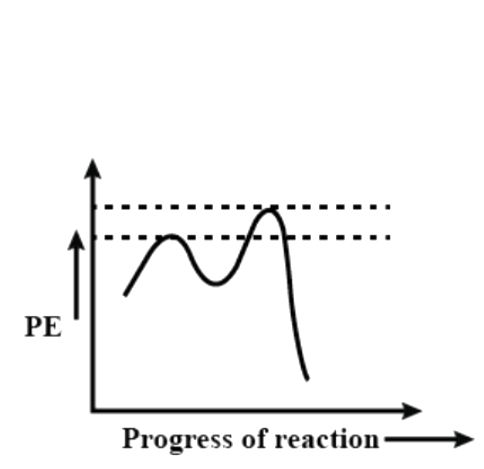
(B)
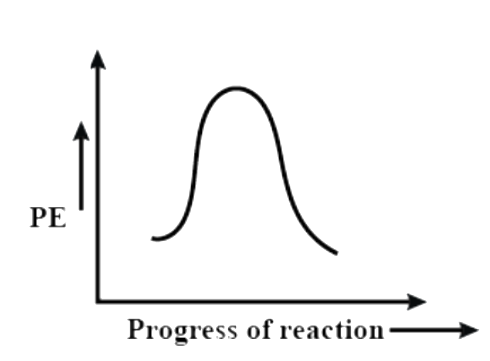
(C)
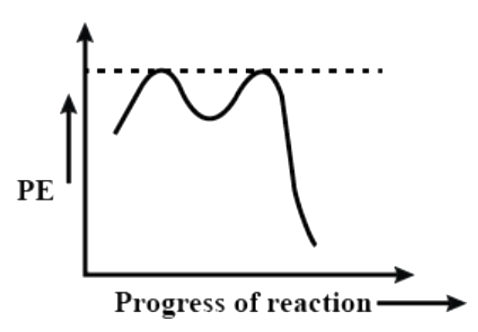
(D)





Answer
577.8k+ views
HINT: To solve this, remember that ${{S}_{N}}1$ is an abbreviation for substitution nucleophilic unimolecular reaction. It is not a concerted mechanism and it takes place in two differentiable steps. Removal of the leaving group and addition of nucleophiles are the main steps.
COMPLETE STEP BY STEP SOLUTION: Before answering this question, let us discuss what ${{S}_{N}}1$ reaction is and also its mechanism. We know that ${{S}_{N}}1$ mechanism is nucleophilic substitution unimolecular reaction. In ${{S}_{N}}1$ reaction, SN basically stands for nucleophilic substitution and 1 stands for unimolecular. In ${{S}_{N}}1$ , the first step i.e. leaving of the leaving group and formation of the carbocation is the slow step and thus the rate determining step and the second step i.e. the attack of the nucleophile is the fast step. Now, let us see the reaction mechanism by taking an example. In the first step of the mechanism, the leaving group leaves the substrate and this leads to the formation of a carbocation. We can write the reaction as-

Then, the nucleophile (Nu) attacks the carbocation and gives us the product.
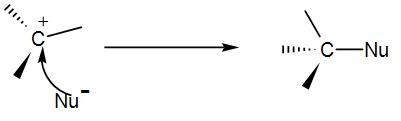
As we can see from the above example, this is a two-step process. The reactivity of compounds undergoing ${{S}_{N}}1$ depends upon 2 factors.
- The first factor is the effectiveness of the removal of the leaving group.
- The second factor is whether or not the carbocation formed is stable.
Now let us see the potential energy diagram of the reaction. We can see from the above example that a carbocation intermediate is formed during the course of the reaction. So, basically the reaction proceeds from reactant to a transition state to intermediate to a second transition state and then product. So, there are two peaks (as we can already understand it is a 2 step reaction). The first transition state includes the bond cleavage of C – X and the second transition state is the formation of C – Nu bond. The intermediate is the carbocation that is formed upon removal of the leaving group. The first transition state peak is higher than the second because it involves the breaking of a C – X bond that is the stable.
So, among the given options, the correct answer is option – (D).
NOTE: Like the nucleophilic substitution we discussed above, there are more substitution reactions. Electrophilic substitution is a reaction where the leaving group is substituted by an electrophile and nucleophilic substitution is a substitution reaction where the leaving group is replaced by the nucleophile by attacking on the positively charged atom to which the leaving group is attached. ${{S}_{N}}2$ mechanism is known as nucleophilic substitution bimolecular. Here, 2 stands for bi-molecular and SN stands for nucleophilic substitution as usual. This mechanism is concerted i.e. the removal of the leaving group and addition of the nucleophile takes place in a single step and the reaction passes through a transition state.
COMPLETE STEP BY STEP SOLUTION: Before answering this question, let us discuss what ${{S}_{N}}1$ reaction is and also its mechanism. We know that ${{S}_{N}}1$ mechanism is nucleophilic substitution unimolecular reaction. In ${{S}_{N}}1$ reaction, SN basically stands for nucleophilic substitution and 1 stands for unimolecular. In ${{S}_{N}}1$ , the first step i.e. leaving of the leaving group and formation of the carbocation is the slow step and thus the rate determining step and the second step i.e. the attack of the nucleophile is the fast step. Now, let us see the reaction mechanism by taking an example. In the first step of the mechanism, the leaving group leaves the substrate and this leads to the formation of a carbocation. We can write the reaction as-

Then, the nucleophile (Nu) attacks the carbocation and gives us the product.

As we can see from the above example, this is a two-step process. The reactivity of compounds undergoing ${{S}_{N}}1$ depends upon 2 factors.
- The first factor is the effectiveness of the removal of the leaving group.
- The second factor is whether or not the carbocation formed is stable.
Now let us see the potential energy diagram of the reaction. We can see from the above example that a carbocation intermediate is formed during the course of the reaction. So, basically the reaction proceeds from reactant to a transition state to intermediate to a second transition state and then product. So, there are two peaks (as we can already understand it is a 2 step reaction). The first transition state includes the bond cleavage of C – X and the second transition state is the formation of C – Nu bond. The intermediate is the carbocation that is formed upon removal of the leaving group. The first transition state peak is higher than the second because it involves the breaking of a C – X bond that is the stable.
So, among the given options, the correct answer is option – (D).
NOTE: Like the nucleophilic substitution we discussed above, there are more substitution reactions. Electrophilic substitution is a reaction where the leaving group is substituted by an electrophile and nucleophilic substitution is a substitution reaction where the leaving group is replaced by the nucleophile by attacking on the positively charged atom to which the leaving group is attached. ${{S}_{N}}2$ mechanism is known as nucleophilic substitution bimolecular. Here, 2 stands for bi-molecular and SN stands for nucleophilic substitution as usual. This mechanism is concerted i.e. the removal of the leaving group and addition of the nucleophile takes place in a single step and the reaction passes through a transition state.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




