
Which of the following pairs give a positive Tollens test?
(A) Glucose, Sucrose
(B) Glucose, Fructose
(C) Hexanal, acetophenone
(D) Fructose, sucrose
Answer
577.5k+ views
Hint: To solve this question we should be aware of the composition of Tollen's Reagent and why the test is performed. The silver mirror coating of the test tube after performing the test indicates that the test is positive.
Complete step by step solution:
Tollen's reagent is an ammoniacal solution of silver oxide. It is obtained by adding ammonia to a precipitate of silver oxide present in a solution of silver nitrate and sodium hydroxide. While preparing the Tollen's reagent make sure that the reagent is well applied to sides of the test tube as this results in formation of a beautiful silver mirror if the Tollens test performed is positive.
Let me explain option wise
-Option A is glucose and sucrose. Firstly, let's draw the structure of both glucose and sucrose:
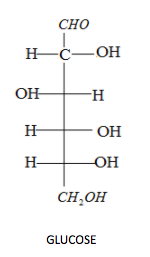
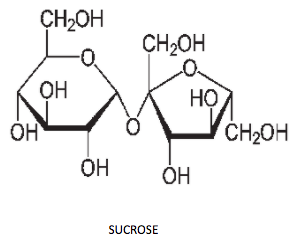
The Tollen's test gives positive results only when aldehyde and $\alpha - hydroxy$ ketone is present. Glucose contains an aldehyde group on top so glucose gives a positive test, but sucrose does not have any free aldehyde or $\alpha - hydroxy$ ketone. Therefore, option A is the wrong answer.
-Option B is Glucose and fructose. Let's draw the structure of both glucose and fructose:
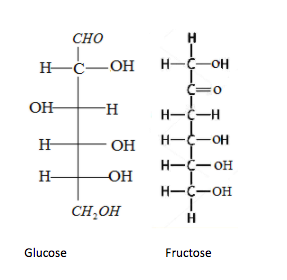
Glucose contains an aldehyde group and fructose contains a $\alpha - hydroxy$ ketone. Tollen's test gives positive results only when aldehyde and $\alpha - hydroxy$ ketone is present. The Tollen's reagent oxidises the aldehyde or $\alpha - hydroxy$ ketone and the silver ions is reduced to metallic forms which get deposited on the sides of the test tube. Therefore option B is the correct answer.
-Option C is hexanal and acetophenone. The hexanal and acetophenone are aldehydes and ketones respectively. Only aldehyde answers for the test, but acetophenone won't answer. Hence option C is wrong.
-Option D is Fructose and sucrose. Let's draw the structure of both fructose and sucrose.
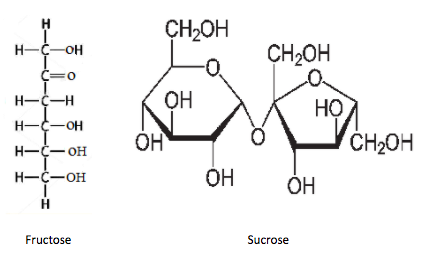
As we saw earlier, fructose will show positive test and sucrose won't show positive test.
Therefore, option D is the wrong option answer.
Thus, option B is the correct answer.
Note: When aldehyde and $\alpha - hydroxy$ ketone is present. Tollen's reagent oxidises the aldehyde or $\alpha - hydroxy$ ketone and the silver ions is reduced to metallic forms which get deposited on the sides of the test tube.
Complete step by step solution:
Tollen's reagent is an ammoniacal solution of silver oxide. It is obtained by adding ammonia to a precipitate of silver oxide present in a solution of silver nitrate and sodium hydroxide. While preparing the Tollen's reagent make sure that the reagent is well applied to sides of the test tube as this results in formation of a beautiful silver mirror if the Tollens test performed is positive.
Let me explain option wise
-Option A is glucose and sucrose. Firstly, let's draw the structure of both glucose and sucrose:


The Tollen's test gives positive results only when aldehyde and $\alpha - hydroxy$ ketone is present. Glucose contains an aldehyde group on top so glucose gives a positive test, but sucrose does not have any free aldehyde or $\alpha - hydroxy$ ketone. Therefore, option A is the wrong answer.
-Option B is Glucose and fructose. Let's draw the structure of both glucose and fructose:

Glucose contains an aldehyde group and fructose contains a $\alpha - hydroxy$ ketone. Tollen's test gives positive results only when aldehyde and $\alpha - hydroxy$ ketone is present. The Tollen's reagent oxidises the aldehyde or $\alpha - hydroxy$ ketone and the silver ions is reduced to metallic forms which get deposited on the sides of the test tube. Therefore option B is the correct answer.
-Option C is hexanal and acetophenone. The hexanal and acetophenone are aldehydes and ketones respectively. Only aldehyde answers for the test, but acetophenone won't answer. Hence option C is wrong.
-Option D is Fructose and sucrose. Let's draw the structure of both fructose and sucrose.

As we saw earlier, fructose will show positive test and sucrose won't show positive test.
Therefore, option D is the wrong option answer.
Thus, option B is the correct answer.
Note: When aldehyde and $\alpha - hydroxy$ ketone is present. Tollen's reagent oxidises the aldehyde or $\alpha - hydroxy$ ketone and the silver ions is reduced to metallic forms which get deposited on the sides of the test tube.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




