
Which of the following options is incorrect?
A. No of positional isomers of G is 2
B. E on heating in the presence of Se gives an aromatic compound
C. D forms a blue color solution in the Victor Meyer test.
D. No of ${2^0}$ carbons in E is 8

Answer
542.4k+ views
Hint: As we know that the Reimer Tiemann reaction is a substitution reaction which is used for ortho-formylation of phenols. In this reaction a phenol is treated with chloroform in presence of potassium hydroxide. It results in the formation of an aldehyde group at the ortho position of the benzene ring that leads to ortho-hydroxybenzaldehyde.
Complete step by step answer:
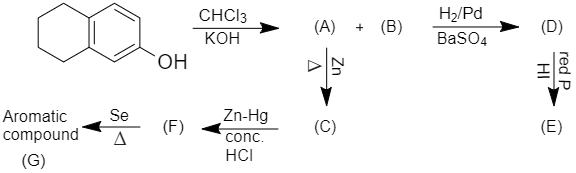
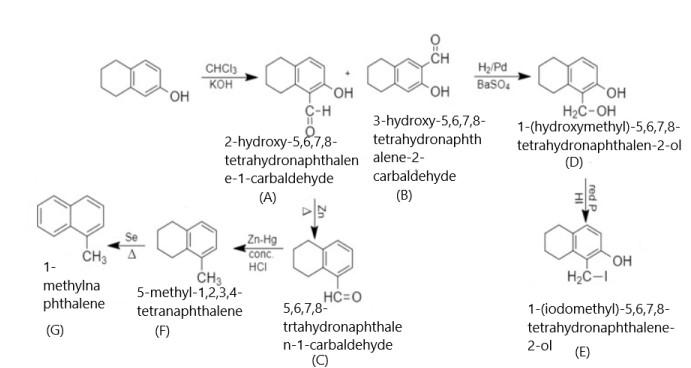
When 5,6,7,8 - tetrahydronaphthalene - 2 - ol is treated with chloroform in presence of potassium hydroxide, it results in the formation of 2 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde and 3 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 2 - carbaldehyde. When 2 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde is reduced with Hydrogen/Palladium, it results in the formation of 1 - (hydroxymethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol.
In this aldehyde is reduced into an alcohol in presence of hydrogen and Palladium. After that, 1 - (hydroxymethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol is treated with Phosphorus and hydrogen iodide and results in the formation of 1 - (iodomethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol.
Now, when 2 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde is heated with zinc, it removes hydroxyl group and results in the formation of 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde. When this product is reacted with zinc amalgam in presence of concentrated HCl, it results in the formation of 5 - methyl - 1,2,3,4 - tetrahydronaphthalene. After that, this product is heated in presence of Selenium and results in the formation of 1 - methylnaphthalene. Thus the whole reaction will be given as under
Let us discuss the given options one by one.
Number of positional isomers of G, that is, methyl naphthalene is two. Therefore, this option is correct.
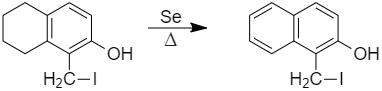
E, that is, 1 - (iodomethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol is heated with Selenium, it gives an aromatic compound.
So, this option is also correct.
D, that is, 1 - (hydroxymethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol does not form a blue color solution in the Victor Meyer test. This is because D is a primary alcohol which forms a red color solution in Victor Meyer test. Therefore, this option is incorrect.
Number of ${2^0}$ carbons in E, 1 - (iodomethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol, is 8. Therefore, this option is correct.
So, the correct answer is Option C.
Note: So, we can see that much more energy is released when we make $MgC{l_2}$ than when we make MgCl. As we need to put in more energy to ionize the magnesium to give a 2 + ion. Using the Born-Haber cycle for lattice enthalpy is a good way of judging how purely ionic is any crystal.
Complete step by step answer:
When 5,6,7,8 - tetrahydronaphthalene - 2 - ol is treated with chloroform in presence of potassium hydroxide, it results in the formation of 2 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde and 3 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 2 - carbaldehyde. When 2 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde is reduced with Hydrogen/Palladium, it results in the formation of 1 - (hydroxymethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol.
In this aldehyde is reduced into an alcohol in presence of hydrogen and Palladium. After that, 1 - (hydroxymethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol is treated with Phosphorus and hydrogen iodide and results in the formation of 1 - (iodomethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol.
Now, when 2 - hydroxy - 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde is heated with zinc, it removes hydroxyl group and results in the formation of 5,6,7,8 - tetrahydronaphthalene - 1 - carbaldehyde. When this product is reacted with zinc amalgam in presence of concentrated HCl, it results in the formation of 5 - methyl - 1,2,3,4 - tetrahydronaphthalene. After that, this product is heated in presence of Selenium and results in the formation of 1 - methylnaphthalene. Thus the whole reaction will be given as under

Let us discuss the given options one by one.
Number of positional isomers of G, that is, methyl naphthalene is two. Therefore, this option is correct.

E, that is, 1 - (iodomethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol is heated with Selenium, it gives an aromatic compound.

So, this option is also correct.
D, that is, 1 - (hydroxymethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol does not form a blue color solution in the Victor Meyer test. This is because D is a primary alcohol which forms a red color solution in Victor Meyer test. Therefore, this option is incorrect.
Number of ${2^0}$ carbons in E, 1 - (iodomethyl) - 5,6,7,8 - tetrahydronaphthalen - 2 - ol, is 8. Therefore, this option is correct.
So, the correct answer is Option C.
Note: So, we can see that much more energy is released when we make $MgC{l_2}$ than when we make MgCl. As we need to put in more energy to ionize the magnesium to give a 2 + ion. Using the Born-Haber cycle for lattice enthalpy is a good way of judging how purely ionic is any crystal.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




