
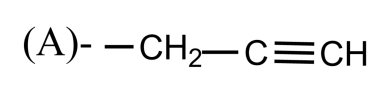
Which of the following is the propargyl group?
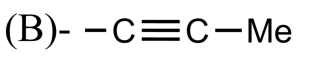
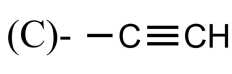
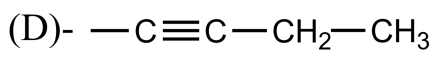

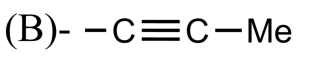
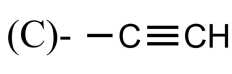
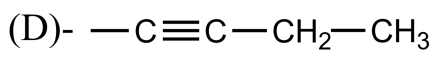
Answer
589.2k+ views
Hint: An alkyl functional group of 2-propynyl is called Propargyl. It is derived from the alkyne propyne.
Complete step by step answer:
-The term propargylic designates to a saturated position ($s{{p}^{3}}$-hybridized ) on a molecular framework that is next to an alkynyl group.
-In simple words, we can say the propargyl group is a portion of the molecular structure which is equivalent to propyne minus one hydrogen atom from carbon 3.
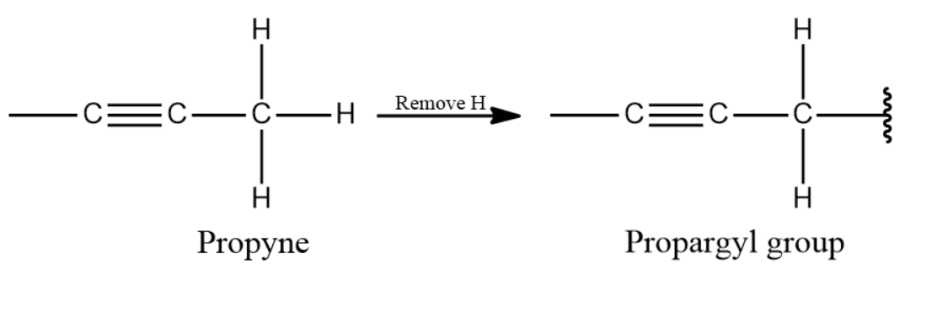
-Propargylic word is a mixture of propene and Argentum and refers to the typical reaction of the terminal alkynes with silver salts.
-The chemical structure of the propargyl group is shown below-
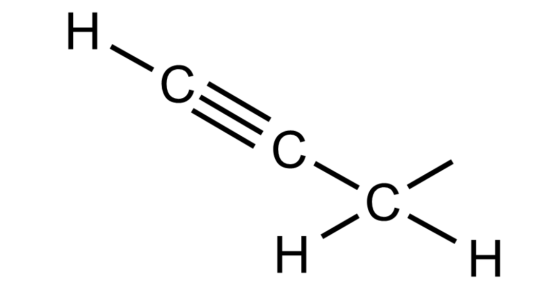
-The term homopropargyl refers to a saturated position on a molecular framework that is next to a propargylic group. The propargylic position in a molecule is a position next to an alkyne group.
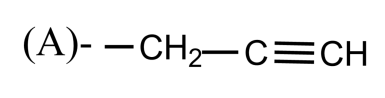
So, the correct answer is “Option A”.
Additional Information:
-Propargyl bromide produced by the treatment of propargyl alcohol with phosphorus tribromide was first used soil fumigant called Trizone.
-Propargyl bromide can also be used as an intermediate for the synthesis of agrochemicals and pharmaceuticals. For example, at low temperatures, it forms a Grignard reagent.
-Iodocyclization reactions are atoms and step economic reactions. Iodocyclization reactions are promoted by simple and readily available inexpensive electrophilic iodine reagents at ambient temperature. Iodocyclization of propargyl alcohols is an active site for the construction of hetero iodides or carbocyclic iodides.
Note: Propargyl esters are effective protective groups for the carboxyl group during solution-phase peptide synthesis. The propargyl ester groups can be introduced onto free amino acids by reacting them with propargyl alcohol saturated with HCl.
Complete step by step answer:
-The term propargylic designates to a saturated position ($s{{p}^{3}}$-hybridized ) on a molecular framework that is next to an alkynyl group.
-In simple words, we can say the propargyl group is a portion of the molecular structure which is equivalent to propyne minus one hydrogen atom from carbon 3.

-Propargylic word is a mixture of propene and Argentum and refers to the typical reaction of the terminal alkynes with silver salts.
-The chemical structure of the propargyl group is shown below-

-The term homopropargyl refers to a saturated position on a molecular framework that is next to a propargylic group. The propargylic position in a molecule is a position next to an alkyne group.
So, the correct answer is “Option A”.

Additional Information:
-Propargyl bromide produced by the treatment of propargyl alcohol with phosphorus tribromide was first used soil fumigant called Trizone.
-Propargyl bromide can also be used as an intermediate for the synthesis of agrochemicals and pharmaceuticals. For example, at low temperatures, it forms a Grignard reagent.
-Iodocyclization reactions are atoms and step economic reactions. Iodocyclization reactions are promoted by simple and readily available inexpensive electrophilic iodine reagents at ambient temperature. Iodocyclization of propargyl alcohols is an active site for the construction of hetero iodides or carbocyclic iodides.
Note: Propargyl esters are effective protective groups for the carboxyl group during solution-phase peptide synthesis. The propargyl ester groups can be introduced onto free amino acids by reacting them with propargyl alcohol saturated with HCl.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




