
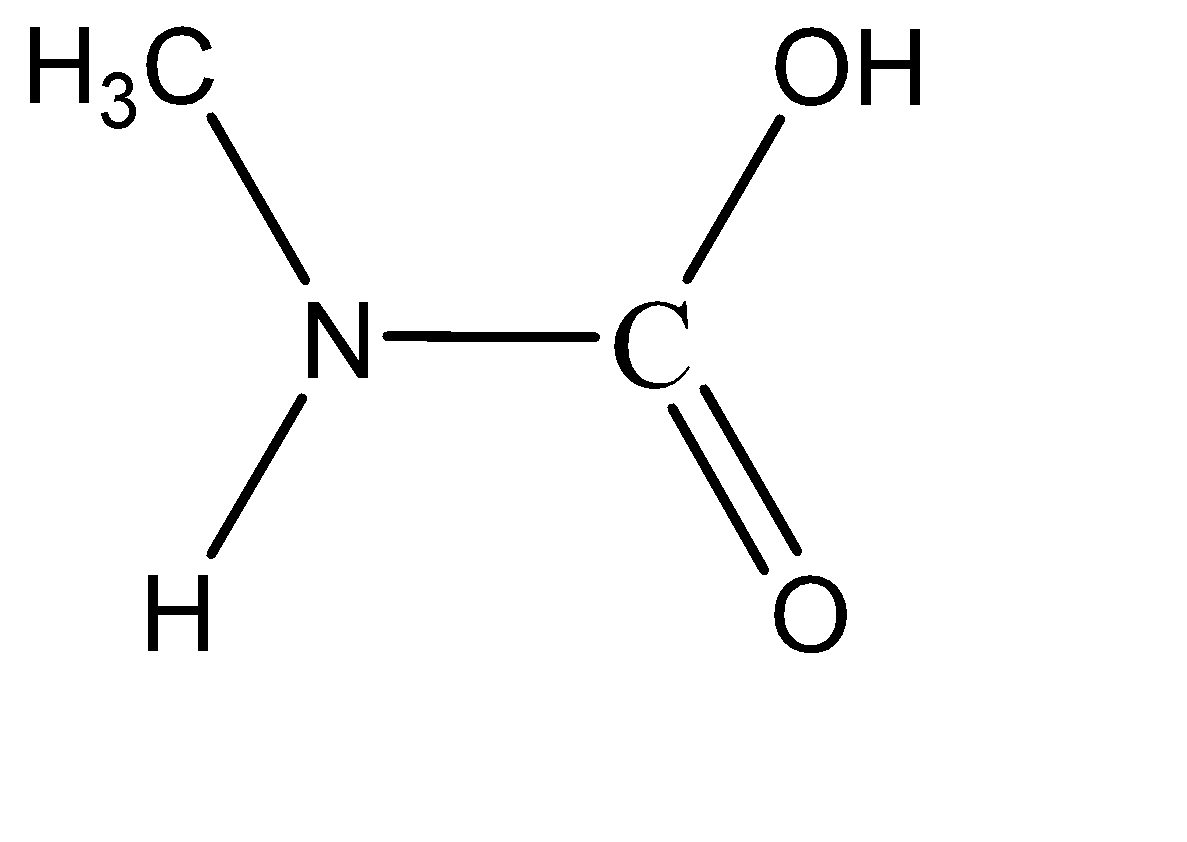
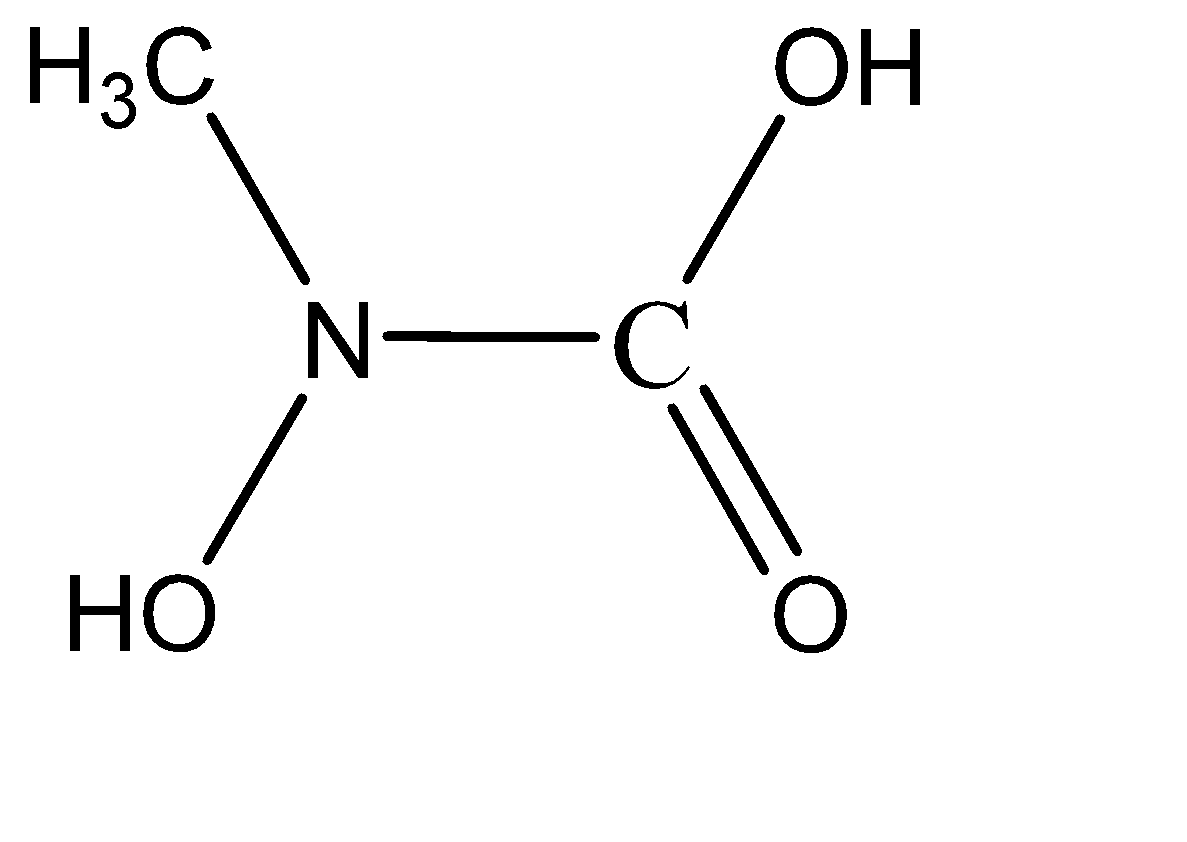
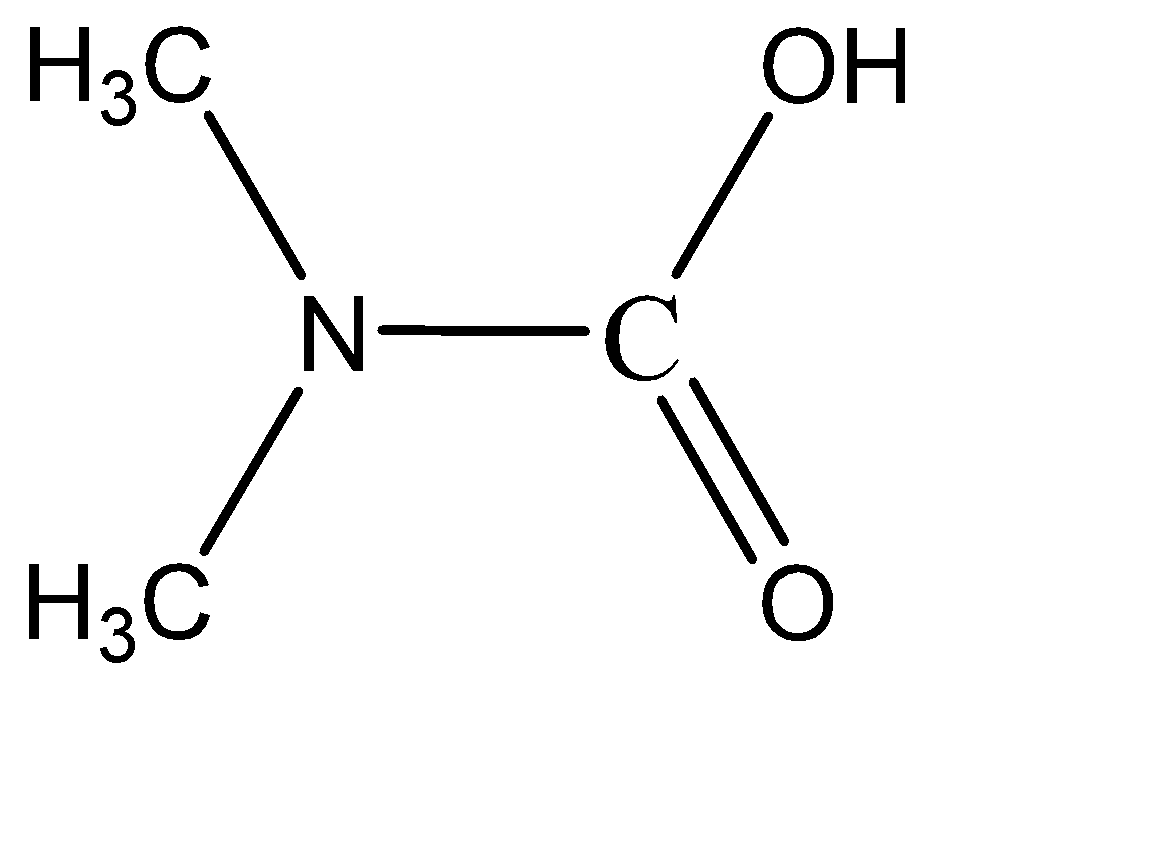
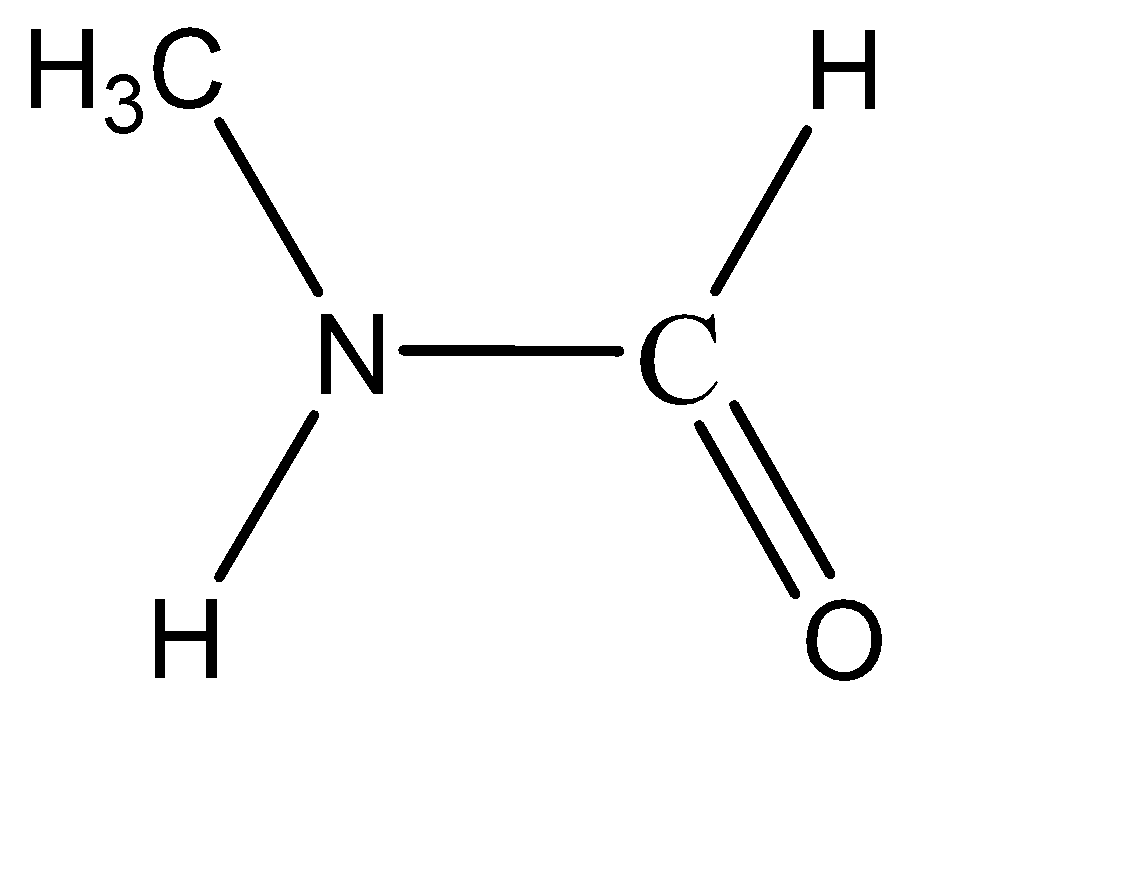
Which of the following is the product of addition of $ {H_2}O $ to methyl isocyanate $ C{H_3} - N = C = O $ ?
A)
B)
C)
D)




Answer
481.5k+ views
Hint: Isocyanate is a functional group with chemical formula $ R - N = C = O $ . The compound which contains this functional group is called isocyanates. Compounds with two isocyanate groups are known as diisocyanate. Isocyanates are highly reactive and low molecular weight compounds used for the manufacture of rigid foams, fibres, etc.
Complete Step By Step Answer:
We are given an additional reaction of $ {H_2}O $ and Methyl Isocyanate. Here water acts as an Nucleophile which attacks the carbon of the isocyanate. There are two proton transfers which give us the final product. The reaction gives amines and carbon dioxide proceeding via a formation of an intermediate.
The mechanism of the reaction of isocyanates with water can be shown as:
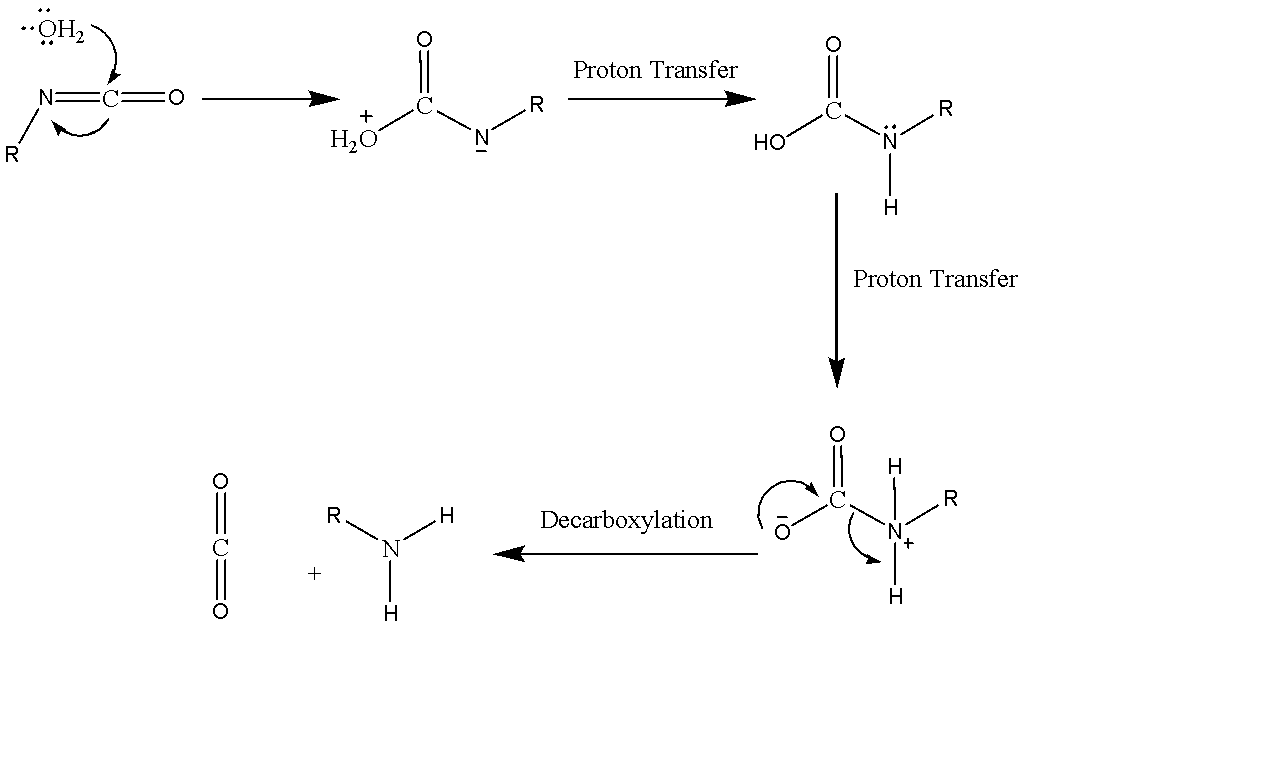
We are given Methyl Isocyanate. The reaction of Methyl Isocyanate will proceed the same way as above, but we need the intermediate product formed before decarboxylation. The complete reaction can be given as:
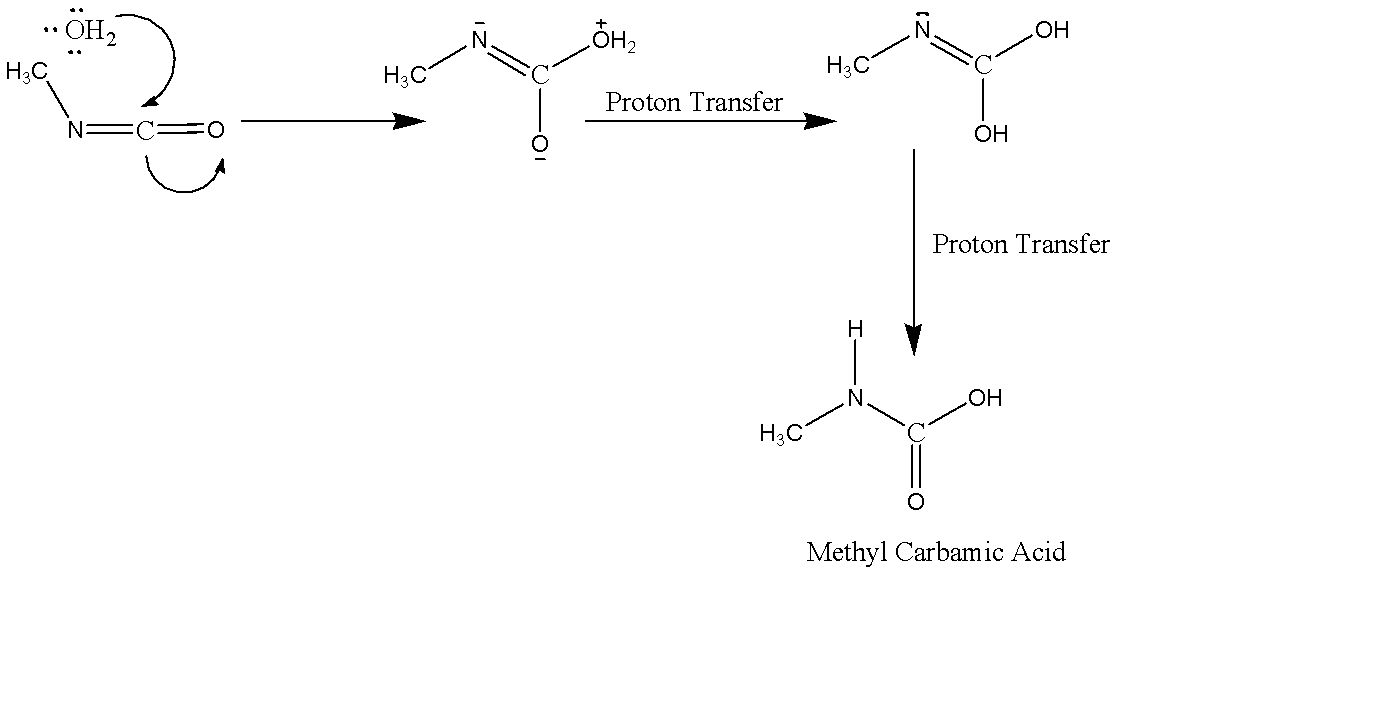
Here Water acts as a Nucleophile which attacks on the Carbon Centre. With two proton transfers it forms Methyl Carbamic acid and further on decarboxylation gives primary amine $ C{H_3} - N{H_2} $ and carbon dioxide.
Hence the correct answer is Option (A).
Note:
The reaction $ R - N = C = O + {H_2}O \to RN{H_2} + C{O_2} $ is used in industries for the production of polyurethane to give polyurethane foams. Here Carbon dioxide is used as a Blowing Agent. The methyl isocyanate is the gas that caused the Bhopal Gas Tragedy. Methyl isocyanate is the smallest molecule of the isocyanate family and is also the most dangerous. It evaporates quickly when exposed to air and has a strong sharp odour.
Complete Step By Step Answer:
We are given an additional reaction of $ {H_2}O $ and Methyl Isocyanate. Here water acts as an Nucleophile which attacks the carbon of the isocyanate. There are two proton transfers which give us the final product. The reaction gives amines and carbon dioxide proceeding via a formation of an intermediate.
The mechanism of the reaction of isocyanates with water can be shown as:

We are given Methyl Isocyanate. The reaction of Methyl Isocyanate will proceed the same way as above, but we need the intermediate product formed before decarboxylation. The complete reaction can be given as:

Here Water acts as a Nucleophile which attacks on the Carbon Centre. With two proton transfers it forms Methyl Carbamic acid and further on decarboxylation gives primary amine $ C{H_3} - N{H_2} $ and carbon dioxide.
Hence the correct answer is Option (A).
Note:
The reaction $ R - N = C = O + {H_2}O \to RN{H_2} + C{O_2} $ is used in industries for the production of polyurethane to give polyurethane foams. Here Carbon dioxide is used as a Blowing Agent. The methyl isocyanate is the gas that caused the Bhopal Gas Tragedy. Methyl isocyanate is the smallest molecule of the isocyanate family and is also the most dangerous. It evaporates quickly when exposed to air and has a strong sharp odour.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




