
Which of the following is the functional group of aldehydes?
(A)
(B)
(C)
(D) None of the above



Answer
515.1k+ views
Hint :A functional group is a group of atoms or bonds within a compound that is responsible for the compound's characteristic chemical reactions in organic chemistry. Regardless of the compound in which it is found, the same functional group can behave similarly and undergo identical reactions.
Complete Step By Step Answer:
A functional group is a substituent or moiety in a molecule that activates the molecule's characteristic chemical reactions in organic chemistry. Regardless of the remainder of the molecule's composition, the same functional group will undergo the same or identical chemical reactions.
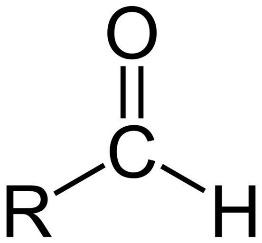
An aldehyde is a chemical compound that contains a functional group with the form - CHO, which consists of a carbonyl centre bonded to hydrogen as well as some generic alkyl or side chain R group. An aldehyde or formal group is the functional group itself.
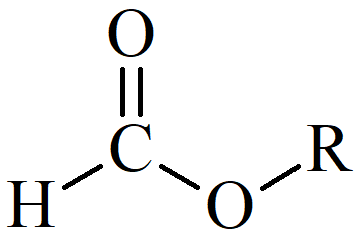
Esters (-COOR`) are a kind of functional group that is often used in organic chemistry. A carbon atom is bound to three other atoms: a single bond to another carbon, a double bond to an oxygen, and a single bond to an oxygen. At the carbonyl carbon, esters react with nucleophiles.
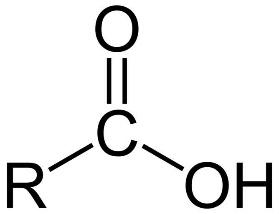
The carbonyl (C=O) and hydroxyl groups combine to form the carboxyl (COOH) group. Aldehydes, ketones, and carboxylic acids are formed as alcohols are oxidised. As organic compounds are oxidised, the number of bonds between carbon and oxygen increases, while the number of bonds between carbon and hydrogen decreases.
Hence option A is correct.
Note :
A carbonyl group is a functional group in organic chemistry that consists of a carbon atom double-bonded to an oxygen atom: C=O. It's found in a variety of organic molecules, and it's a component of much broader functional groups. A carbonyl compound is a chemical compound that contains a carbonyl group.
Complete Step By Step Answer:
A functional group is a substituent or moiety in a molecule that activates the molecule's characteristic chemical reactions in organic chemistry. Regardless of the remainder of the molecule's composition, the same functional group will undergo the same or identical chemical reactions.
An aldehyde is a chemical compound that contains a functional group with the form - CHO, which consists of a carbonyl centre bonded to hydrogen as well as some generic alkyl or side chain R group. An aldehyde or formal group is the functional group itself.
Esters (-COOR`) are a kind of functional group that is often used in organic chemistry. A carbon atom is bound to three other atoms: a single bond to another carbon, a double bond to an oxygen, and a single bond to an oxygen. At the carbonyl carbon, esters react with nucleophiles.
The carbonyl (C=O) and hydroxyl groups combine to form the carboxyl (COOH) group. Aldehydes, ketones, and carboxylic acids are formed as alcohols are oxidised. As organic compounds are oxidised, the number of bonds between carbon and oxygen increases, while the number of bonds between carbon and hydrogen decreases.
Hence option A is correct.
Note :
A carbonyl group is a functional group in organic chemistry that consists of a carbon atom double-bonded to an oxygen atom: C=O. It's found in a variety of organic molecules, and it's a component of much broader functional groups. A carbonyl compound is a chemical compound that contains a carbonyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




