
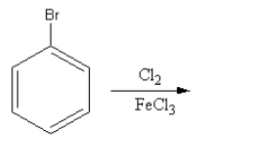
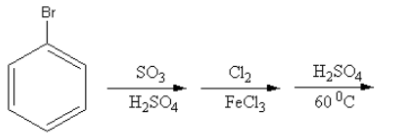
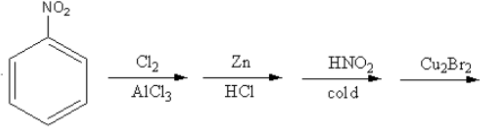
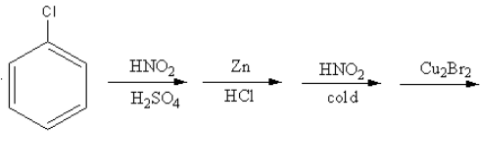
Which of the following is the best method for the synthesis of $1 - $bromo$ - 3 - $chloro benzene?
A)
B)
C)
D)




Answer
578.1k+ views
Hint: We can decide the site of chlorination by checking the directing effect of the halogen and nitro group. The nitro group shows \[\] effect and halogens $ + {\text{M}}\,$show effect.
Complete step by step answer:
The synthesis of the product $1 - $bromo$ - 3 - $chloro benzene depends upon the directing effect of the substituent attached at the benzene ring.
The addition of chlorine is known as chlorination. The chlorination can occur at any position in the benzene ring. But if any group is present on the benzene ring the group causes the partial charge separation, so it directs the attacking group according to its nature on the benzene ring.
${\text{Cl}}$ and ${\text{Br}}$ are ortho-para directing groups. They show $ + {\text{M}}\,$ effect so they donate the electrons towards the benzene ring thus, increase the electron density at ortho and para position.
So, electrophile chlorine attacks at the ortho and para position so, the product of the reaction will be $2 - $chloro or \[4 - \]chloro.
Nitro is a meta directing group. It shows \[ - {\text{M}}\,\] effect so it attracts the electrons towards itself thus, increasing the electron density on meta position.
So, electrophile chlorine attacks at the meta position, so the product of the reaction will be $1 - $bromo$ - 3 - $chloro.
The reaction of chlorination is as follows:
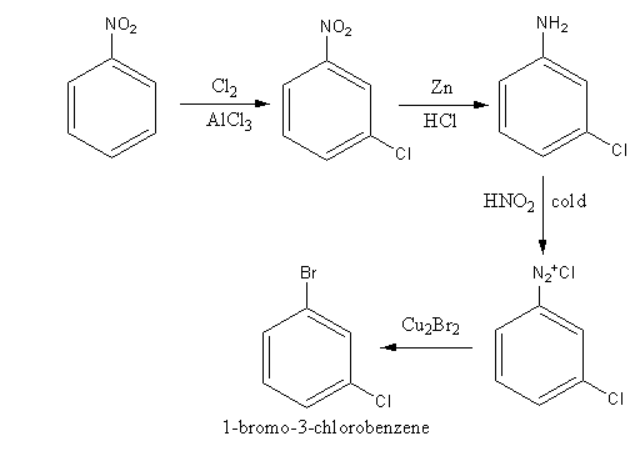
Therefore, option (C) is correct.
Note:
The groups that withdraw the electron density from the ring show the \[ - {\text{M}}\,\] effect and work as deactivating, meta directing whereas the groups that donate the electron density to the ring show the ${\text{ + M}}$ effect and work as activating ortho and para directing. The halogens are ortho-para directing and deactivating because halogen shows $ - {\text{I}}$.
Complete step by step answer:
The synthesis of the product $1 - $bromo$ - 3 - $chloro benzene depends upon the directing effect of the substituent attached at the benzene ring.
The addition of chlorine is known as chlorination. The chlorination can occur at any position in the benzene ring. But if any group is present on the benzene ring the group causes the partial charge separation, so it directs the attacking group according to its nature on the benzene ring.
${\text{Cl}}$ and ${\text{Br}}$ are ortho-para directing groups. They show $ + {\text{M}}\,$ effect so they donate the electrons towards the benzene ring thus, increase the electron density at ortho and para position.
So, electrophile chlorine attacks at the ortho and para position so, the product of the reaction will be $2 - $chloro or \[4 - \]chloro.
Nitro is a meta directing group. It shows \[ - {\text{M}}\,\] effect so it attracts the electrons towards itself thus, increasing the electron density on meta position.
So, electrophile chlorine attacks at the meta position, so the product of the reaction will be $1 - $bromo$ - 3 - $chloro.
The reaction of chlorination is as follows:

Therefore, option (C) is correct.
Note:
The groups that withdraw the electron density from the ring show the \[ - {\text{M}}\,\] effect and work as deactivating, meta directing whereas the groups that donate the electron density to the ring show the ${\text{ + M}}$ effect and work as activating ortho and para directing. The halogens are ortho-para directing and deactivating because halogen shows $ - {\text{I}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




