
Which of the following is least acidic?
A. $p - nitrophenol$
B. $p - chlorophenol$
C. $phenol$
D. $o - cresol$
Answer
558.9k+ views
Hint: We have to remember that in organic chemistry, an acid is a substance which donates a proton ( ${H^ + }$ , hydrogen ion), by Bronsted-Lowry, so called Bronsted-Lowry acid. And a Lewis acid, accepts an electron pair to form a covalent bond. Other definitions for acid are there but the Bronsted-Lowry definition is mostly used definition for acid.
Complete step by step answer:
We must have to know that the aromatic nucleus contains a hydroxyl group(s) called phenols. It is represented by the general formula ArOH, Ar is a phenyl group $({C_6}{H_5} - )$ .
We can draw the structures of given phenols are,

We must have to know that the fairly acidic compounds are phenols. Phenol acidity is explained by phenoxide ion, which is formed by the loss of hydrogen,
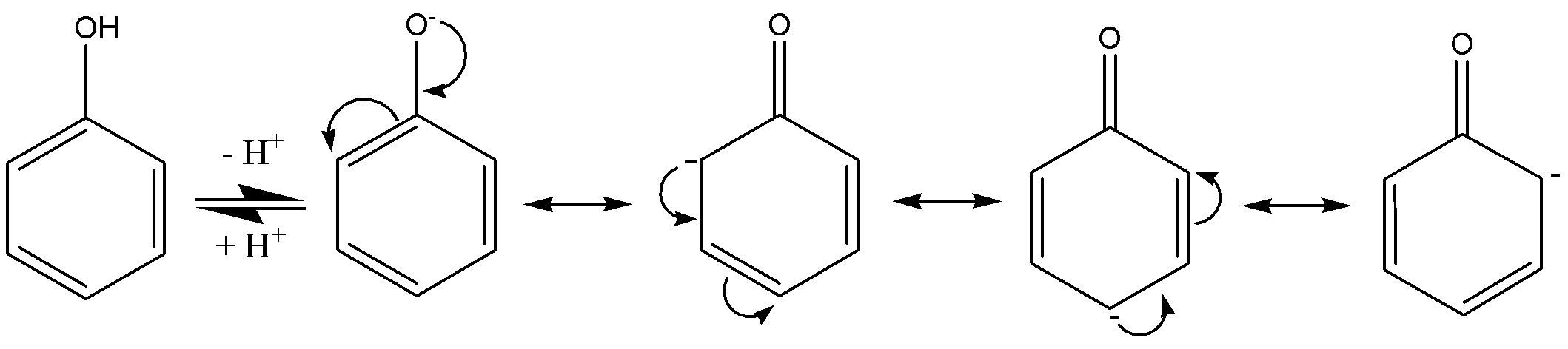
We need to know that if electron-releasing groups (like alkyl) attached in the phenols, then the electron-releasing groups will destabilize the phenoxide ion by intensifying the negative charge through ${e^ - }$ release by (+R) resonance effect and/or (+I) inductive effect. So, the electron-releasing groups on phenols are weakening the strength of acidic character, hence they are weak acids.
In contrast, if electron-withdrawing groups (like $N{O_2},Cl$ ) attached in the phenols, then the electron-withdrawing groups will stabilize the phenoxide ion by charge dispersal through powerful electron-withdrawing (-I) inductive effect. Hence the acids are strong.
Among these options $p - nitrophenol$ , $p - chlorophenol$ indicates that contains electron-withdrawing groups (nitro and chloro), so the acidic character is more compared to $phenol$ and $o - cresol$ .
We can draw the structure of $o - cresol$ (Also known as $o - methylphenol$) structure as,

We have to remember that the $o - cresol$ contains an alkyl group, which is an electron-releasing group, so the alkyl group destabilizes the phenoxide ion by intensifying the negative charge, so this will decrease the acidic character and this is weak acid.
$o - cresol$ is least acidic and it is the correct option from the above information.
Among these four options $p - nitrophenol$, $p - chlorophenol$, $phenol$ and $o - cresol$, $o - cresol$ is least acidic, the order of increasing acidic character among these options are,
$o - cresol$ < $phenol$ < $p - chlorophenol$ < $p - nitrophenol$
$p - nitrophenol$ contains oxygen atoms, which allows more stability than chlorine.
Therefore, option D is correct.
Note:
We need to remember that the benzoylation of any benzene moiety having at least one hydrogen atom bonded to electronegativity atom. Benzoylation in benzene compared to another benzene derivative is less reactive. Because all the hydrogen is having the same electronegativity. Benzoylation is an important reaction for forming larger benzene moieties. In new drug designing purposes, most of the scientists use these techniques for synthesis of new molecules. In this reaction, larger benzene moiety is also applicable.
Complete step by step answer:
We must have to know that the aromatic nucleus contains a hydroxyl group(s) called phenols. It is represented by the general formula ArOH, Ar is a phenyl group $({C_6}{H_5} - )$ .
We can draw the structures of given phenols are,

We must have to know that the fairly acidic compounds are phenols. Phenol acidity is explained by phenoxide ion, which is formed by the loss of hydrogen,

We need to know that if electron-releasing groups (like alkyl) attached in the phenols, then the electron-releasing groups will destabilize the phenoxide ion by intensifying the negative charge through ${e^ - }$ release by (+R) resonance effect and/or (+I) inductive effect. So, the electron-releasing groups on phenols are weakening the strength of acidic character, hence they are weak acids.
In contrast, if electron-withdrawing groups (like $N{O_2},Cl$ ) attached in the phenols, then the electron-withdrawing groups will stabilize the phenoxide ion by charge dispersal through powerful electron-withdrawing (-I) inductive effect. Hence the acids are strong.
Among these options $p - nitrophenol$ , $p - chlorophenol$ indicates that contains electron-withdrawing groups (nitro and chloro), so the acidic character is more compared to $phenol$ and $o - cresol$ .
We can draw the structure of $o - cresol$ (Also known as $o - methylphenol$) structure as,

We have to remember that the $o - cresol$ contains an alkyl group, which is an electron-releasing group, so the alkyl group destabilizes the phenoxide ion by intensifying the negative charge, so this will decrease the acidic character and this is weak acid.
$o - cresol$ is least acidic and it is the correct option from the above information.
Among these four options $p - nitrophenol$, $p - chlorophenol$, $phenol$ and $o - cresol$, $o - cresol$ is least acidic, the order of increasing acidic character among these options are,
$o - cresol$ < $phenol$ < $p - chlorophenol$ < $p - nitrophenol$
$p - nitrophenol$ contains oxygen atoms, which allows more stability than chlorine.
Therefore, option D is correct.
Note:
We need to remember that the benzoylation of any benzene moiety having at least one hydrogen atom bonded to electronegativity atom. Benzoylation in benzene compared to another benzene derivative is less reactive. Because all the hydrogen is having the same electronegativity. Benzoylation is an important reaction for forming larger benzene moieties. In new drug designing purposes, most of the scientists use these techniques for synthesis of new molecules. In this reaction, larger benzene moiety is also applicable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




