
Which of the following is /are an example of orthosilicate? (This question has multiple correct answers)
(a)- $ZrSi{{O}_{4}}$
(b)- $M{{g}_{2}}Si{{O}_{4}}$
(c)- $Z{{n}_{3}}S{{i}_{2}}{{O}_{7}}$
(d)- Asbestos
Answer
533.4k+ views
Hint: Orthosilicates are the group of compounds in the chemistry in which silicate is the anion part in which silicon is attached with four oxygen atoms through a single bond and has a 4- charge. So, check all the compounds, if the compound has ${{[Si{{O}_{4}}]}^{4-}}$ anion, then it is an orthosilicate.
Complete step-by-step answer:
Orthosilicates are also known as silicon tetroxide anion. The structure of the silicate ion in the orthosilicate is given below:
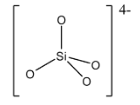
Since, the silicate ion ${{[Si{{O}_{4}}]}^{4-}}$ is the anion part, the cations like sodium, magnesium, etc can form salts with this and are called as orthosilicate.
Let us check all the options.
(a)- $ZrSi{{O}_{4}}$: This compound is known as Zirconium silicate, it is also known as Zirconium orthosilicate because the ions in this are in the form $Z{{r}^{4+}}$ and ${{[Si{{O}_{4}}]}^{4-}}$. The structure is given below:
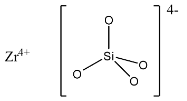
(b)- $M{{g}_{2}}Si{{O}_{4}}$: This compound is magnesium orthosilicate in which magnesium is the cation and ${{[Si{{O}_{4}}]}^{4-}}$ is the anion. The structure is given below:
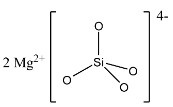
(c)- $Z{{n}_{3}}S{{i}_{2}}{{O}_{7}}$: This compound is zinc pyrosilicate. The pyrosilicate doesn’t have ${{[Si{{O}_{4}}]}^{4-}}$ anion.
(d)- Asbestos: It is also a silicate of magnesium but it is not an orthosilicate because the formula of asbestos is $M{{g}_{3}}S{{i}_{2}}{{O}_{5}}{{(OH)}_{4}}$ .
Therefore, the correct answers are an option (a) and option (b).
Note: It must be noted that not all the silicates are orthosilicate, only those compounds in which the anion is ${{[Si{{O}_{4}}]}^{4-}}$ will be orthosilicate. Since asbestos is used for many purposes but it is a cancer-causing substance.
Complete step-by-step answer:
Orthosilicates are also known as silicon tetroxide anion. The structure of the silicate ion in the orthosilicate is given below:

Since, the silicate ion ${{[Si{{O}_{4}}]}^{4-}}$ is the anion part, the cations like sodium, magnesium, etc can form salts with this and are called as orthosilicate.
Let us check all the options.
(a)- $ZrSi{{O}_{4}}$: This compound is known as Zirconium silicate, it is also known as Zirconium orthosilicate because the ions in this are in the form $Z{{r}^{4+}}$ and ${{[Si{{O}_{4}}]}^{4-}}$. The structure is given below:

(b)- $M{{g}_{2}}Si{{O}_{4}}$: This compound is magnesium orthosilicate in which magnesium is the cation and ${{[Si{{O}_{4}}]}^{4-}}$ is the anion. The structure is given below:

(c)- $Z{{n}_{3}}S{{i}_{2}}{{O}_{7}}$: This compound is zinc pyrosilicate. The pyrosilicate doesn’t have ${{[Si{{O}_{4}}]}^{4-}}$ anion.
(d)- Asbestos: It is also a silicate of magnesium but it is not an orthosilicate because the formula of asbestos is $M{{g}_{3}}S{{i}_{2}}{{O}_{5}}{{(OH)}_{4}}$ .
Therefore, the correct answers are an option (a) and option (b).
Note: It must be noted that not all the silicates are orthosilicate, only those compounds in which the anion is ${{[Si{{O}_{4}}]}^{4-}}$ will be orthosilicate. Since asbestos is used for many purposes but it is a cancer-causing substance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




