
Which of the following is a mixed ketone?
A. Pentanone
B. Acetophenone
C. Benzophenone
D. Butanone
Answer
546.6k+ views
Hint: In a mixed ketone, one part of the compound is alkyl group, or straight chain and the other part of the compound consists of a ring, or aryl group.
Complete answer:
In order to answer the question, we need to learn some facts about the carbonyl group. Now, in the carbonyl group, the carbon is $s{{p}^{2}}$ hybridised. Moreover, the carbon oxygen bond is polar. It is because of the electronegativity difference that is present in the bond. Oxygen is a more electronegative element than carbon and it tends to pull the shared pair of electrons towards itself, whereas carbon is less electronegative than carbon and donates electrons. As a result, the carbon has a partial positive charge and the oxygen exhibits a partial negative charge around it and this is the reason for any nucleophile to attack it.
Now, ketones are of two types, mainly alkyl ketones and aryl ketones. Alkyl ketones are those ketones which have chain structure, whereas in aryl ketones, the ring is present. They can be aromatic too. However, a mixture of these two types are also present where one part of the ketone has a chain and the other part consists of an aromatic ring. Now, let us see the structure of acetophenone in option B:
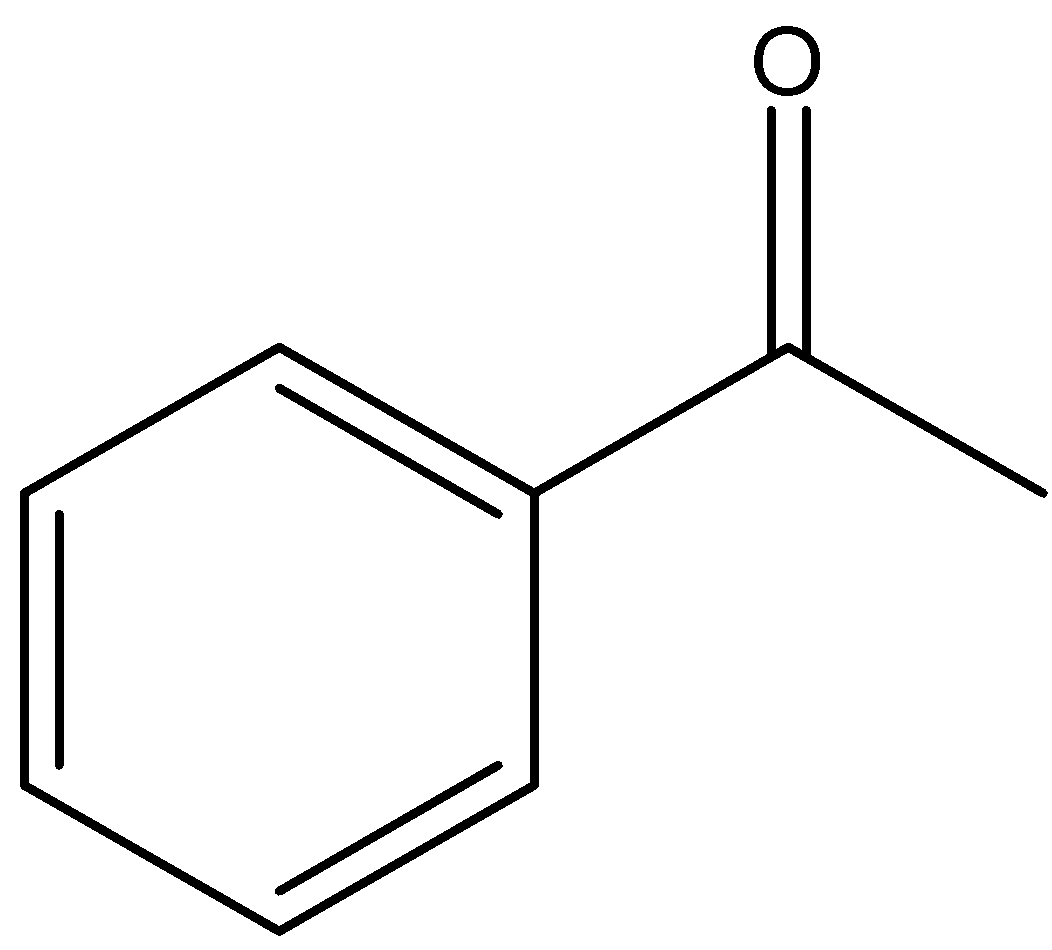
Here, we can see that there is an aromatic ring and an alkyl group both are present. Now, let us see the structures of other ketones in the question:
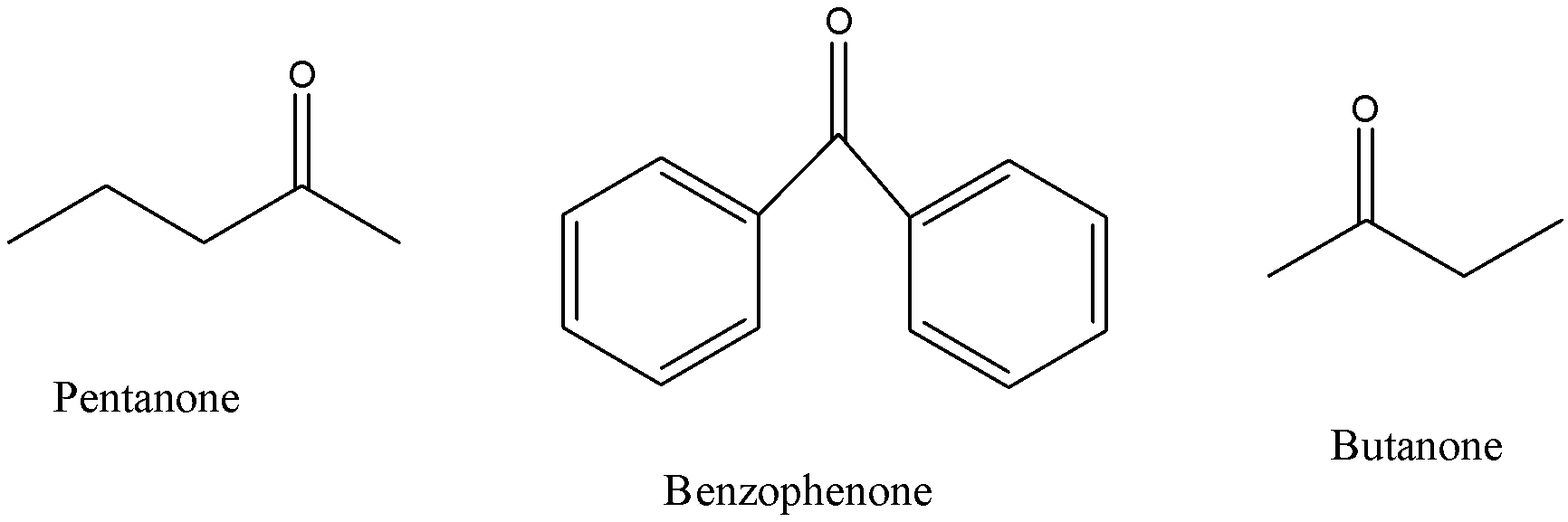
Pentanone and butanone have only chains present, hence they are alkyl ketones, whereas in benzophenone we have two aromatic rings which makes it an aryl ketone.
So, we get option (B) as the correct answer.
Note:
It is to be noted that due to symmetry and resonance in aromatic rings, the aryl ketones are considered to be more stable than alkyl ketones. They also have a high melting and boiling point.
Complete answer:
In order to answer the question, we need to learn some facts about the carbonyl group. Now, in the carbonyl group, the carbon is $s{{p}^{2}}$ hybridised. Moreover, the carbon oxygen bond is polar. It is because of the electronegativity difference that is present in the bond. Oxygen is a more electronegative element than carbon and it tends to pull the shared pair of electrons towards itself, whereas carbon is less electronegative than carbon and donates electrons. As a result, the carbon has a partial positive charge and the oxygen exhibits a partial negative charge around it and this is the reason for any nucleophile to attack it.
Now, ketones are of two types, mainly alkyl ketones and aryl ketones. Alkyl ketones are those ketones which have chain structure, whereas in aryl ketones, the ring is present. They can be aromatic too. However, a mixture of these two types are also present where one part of the ketone has a chain and the other part consists of an aromatic ring. Now, let us see the structure of acetophenone in option B:

Here, we can see that there is an aromatic ring and an alkyl group both are present. Now, let us see the structures of other ketones in the question:

Pentanone and butanone have only chains present, hence they are alkyl ketones, whereas in benzophenone we have two aromatic rings which makes it an aryl ketone.
So, we get option (B) as the correct answer.
Note:
It is to be noted that due to symmetry and resonance in aromatic rings, the aryl ketones are considered to be more stable than alkyl ketones. They also have a high melting and boiling point.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




