
Which of the following is a biodegradable polymer?
(A)
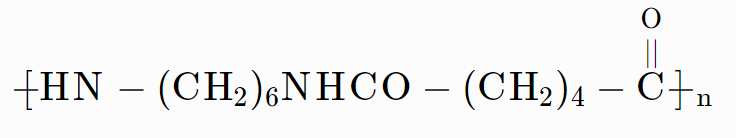
(B)
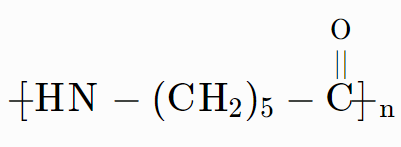
(C)
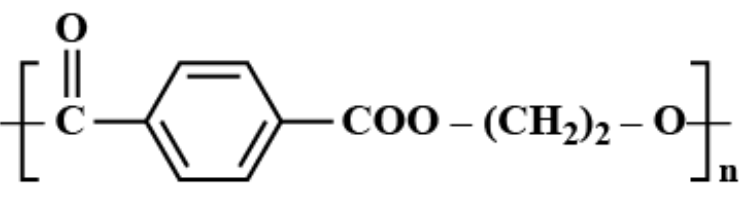
(D)

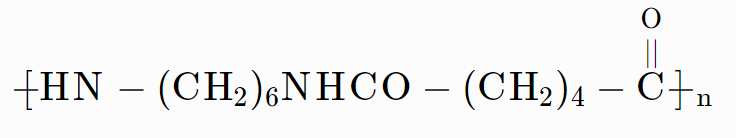
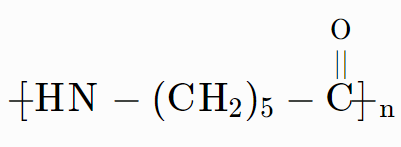

Answer
528.9k+ views
Hint :We know that the polymers are macromolecules built up by linking together a large number of small molecules. The repeating units in a polymer are linked through strong covalent bonds. Small molecules that combine to form polymer molecules are called monomers. The polymers which are decomposed by bacteria are called biodegradable polymers.
Complete Step By Step Answer:
A large number of polymers are responsible for the accumulation of polymeric solid waste materials because these are quite resistant to the environmental degradation process. These polymers have remained underrated for a long time and cause acute environmental problems. Biodegradable synthetic polymers are designed and developed to overcome the problems created by the polymers created by the polymeric solid waste. Similar to functional groups in biopolymers, these biodegradable polymers contain functional groups. One of the important classifications of biodegradable polymers are aliphatic polyesters and some examples are, poly- $ \beta $ -hydroxybutyrate – co- $ \beta $ -hydroxy valerate (PHBV) and $ Nylon\text{ }2-nylon-6.\text{ }Nylon\text{ }2-nylon-6: $ This is an alternating polyamide copolymer of glycine ( $ {{H}_{2}}N-C{{H}_{2}}-COOH $ ) and amino caproic acid ( $ {{H}_{2}}N-{{(C{{H}_{2}})}_{5}}-COOH $ ) is a biodegradable polymer. $ n[{{H}_{2}}N-C{{H}_{2}}-COOH]+n[{{H}_{2}}N-{{(C{{H}_{2}})}_{5}}-COOH]\to {{(-HN-C{{H}_{2}}-CO-HN-{{(C{{H}_{2}})}_{5}}- CO-)}_{n}} $ Hence, Nylon 2-nylon is a biodegradable polymer.
The polymer shown in option (D) is a biodegradable polymer. This polymer is known as $ Nylon-2-nylon-6. $ It is a copolymer of glycine and w-amino caproic acid. It contains polyamide linkages.
Note :
Remember that Buna-S is a synthetic rubber and $ nylon\text{ }-6,\text{ }nylon\text{ }\text{ }6,\text{ }6 $ is synthetic fibers All of these polymers are prepared by condensation polymerization and non-biodegradable polymers. The applications of $ Nylon\text{ }2-nylon-6 $ as a thread in bristles for toothbrushes and making strings of musical instruments. This polymer is also used in the synthesis of artificial fibers.
Complete Step By Step Answer:
A large number of polymers are responsible for the accumulation of polymeric solid waste materials because these are quite resistant to the environmental degradation process. These polymers have remained underrated for a long time and cause acute environmental problems. Biodegradable synthetic polymers are designed and developed to overcome the problems created by the polymers created by the polymeric solid waste. Similar to functional groups in biopolymers, these biodegradable polymers contain functional groups. One of the important classifications of biodegradable polymers are aliphatic polyesters and some examples are, poly- $ \beta $ -hydroxybutyrate – co- $ \beta $ -hydroxy valerate (PHBV) and $ Nylon\text{ }2-nylon-6.\text{ }Nylon\text{ }2-nylon-6: $ This is an alternating polyamide copolymer of glycine ( $ {{H}_{2}}N-C{{H}_{2}}-COOH $ ) and amino caproic acid ( $ {{H}_{2}}N-{{(C{{H}_{2}})}_{5}}-COOH $ ) is a biodegradable polymer. $ n[{{H}_{2}}N-C{{H}_{2}}-COOH]+n[{{H}_{2}}N-{{(C{{H}_{2}})}_{5}}-COOH]\to {{(-HN-C{{H}_{2}}-CO-HN-{{(C{{H}_{2}})}_{5}}- CO-)}_{n}} $ Hence, Nylon 2-nylon is a biodegradable polymer.
The polymer shown in option (D) is a biodegradable polymer. This polymer is known as $ Nylon-2-nylon-6. $ It is a copolymer of glycine and w-amino caproic acid. It contains polyamide linkages.
Note :
Remember that Buna-S is a synthetic rubber and $ nylon\text{ }-6,\text{ }nylon\text{ }\text{ }6,\text{ }6 $ is synthetic fibers All of these polymers are prepared by condensation polymerization and non-biodegradable polymers. The applications of $ Nylon\text{ }2-nylon-6 $ as a thread in bristles for toothbrushes and making strings of musical instruments. This polymer is also used in the synthesis of artificial fibers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




