
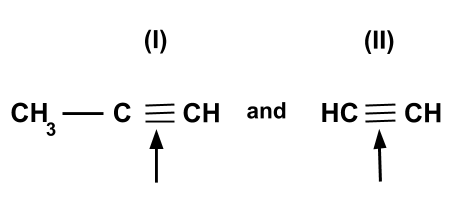
Which of the following indicates a bond has less bond dissociation energy?
A.I
B.II
C.Both are equal
D.Cannot determine

Answer
576k+ views
Hint: There is hyperconjugation in the first compound and in the second compound there is no hyperconjugation. The hyperconjugation occurs when there is presence of carbon – hydrogen sigma bond in the compound.
Complete answer:
-Here, we have to indicate a bond which has less bond dissociation energy, so we have to know about the bond dissociation energy. Bond dissociation energy can be defined as an energy required to break one mole of bond in gaseous state.
-In the first compound we can see that carbon is single bonded with $C{H_3}$ and triple bonded with $CH$ whereas in the second compound there is no carbon in the middle and $CH$ is triple bonded with $CH$.
-Therefore, in the first compound there is hyperconjugation between carbon – carbon triple bond and $\alpha C - H$ sigma bond. Due to the occurrence of hyperconjugation, the triple bond character of first compound is slightly reduced.
-While in the second compound there is no occurrence of hyperconjugation due to which there is no effect in triple bond character. In this compound the hyperconjugation does not occur because of the absence of $\alpha C - H$ sigma bond.
-In the above explanation, we have heard about the word hyperconjugation. So, we should know what hyperconjugation is? So, hyperconjugation is the process of delocalization of electrons which include the participation of sigma bonds. This involves the interaction of electrons in sigma orbitals, for example, $C - H$ or $C - C$ bond with an adjacent unpopulated non – bonding $p$ or anti – bonding ${\sigma ^*}$ or ${\pi ^*}$ orbitals to give pairs of extended molecular orbitals.
Hence, the bond strength of the carbon – carbon triple bond is slightly lower in the first compound than in the second compound.
So, the bond dissociation energy is lower in the first compound than in the second compound. Hence, the correct option is (A).
Note:
When we add substitution to carbon in $C - H$ bond, the bond dissociation energy decreases. The bond dissociation energy of bond $C - H$ is $389kJ/mol$ or $93kcal/mol$.
Complete answer:
-Here, we have to indicate a bond which has less bond dissociation energy, so we have to know about the bond dissociation energy. Bond dissociation energy can be defined as an energy required to break one mole of bond in gaseous state.
-In the first compound we can see that carbon is single bonded with $C{H_3}$ and triple bonded with $CH$ whereas in the second compound there is no carbon in the middle and $CH$ is triple bonded with $CH$.
-Therefore, in the first compound there is hyperconjugation between carbon – carbon triple bond and $\alpha C - H$ sigma bond. Due to the occurrence of hyperconjugation, the triple bond character of first compound is slightly reduced.
-While in the second compound there is no occurrence of hyperconjugation due to which there is no effect in triple bond character. In this compound the hyperconjugation does not occur because of the absence of $\alpha C - H$ sigma bond.
-In the above explanation, we have heard about the word hyperconjugation. So, we should know what hyperconjugation is? So, hyperconjugation is the process of delocalization of electrons which include the participation of sigma bonds. This involves the interaction of electrons in sigma orbitals, for example, $C - H$ or $C - C$ bond with an adjacent unpopulated non – bonding $p$ or anti – bonding ${\sigma ^*}$ or ${\pi ^*}$ orbitals to give pairs of extended molecular orbitals.
Hence, the bond strength of the carbon – carbon triple bond is slightly lower in the first compound than in the second compound.
So, the bond dissociation energy is lower in the first compound than in the second compound. Hence, the correct option is (A).
Note:
When we add substitution to carbon in $C - H$ bond, the bond dissociation energy decreases. The bond dissociation energy of bond $C - H$ is $389kJ/mol$ or $93kcal/mol$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




