
Which of the following exhibit mutarotation?
(this question has multiple correct options)
A.Glucose
B.Maltose
C. Fructose
D. Galactose
Answer
600.6k+ views
Hint: For a compound to show mutarotation, presence of a hemiacetal linkage is an essential requirement. For a hemiacetal linkage, a 6 membered ring with a hydroxyl group is needed.
Complete step by step answer:
Mutarotation is the change in optical rotation because of the change in equilibrium of the two anomers, when corresponding stereocenters interconvert.
First, let us take a look at the structures of all the above-mentioned options:
Glucose –
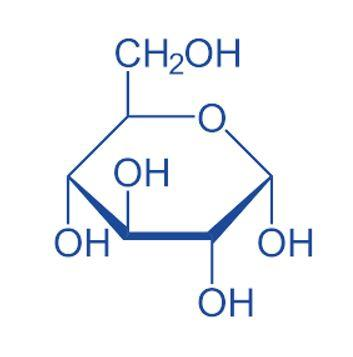
Maltose –
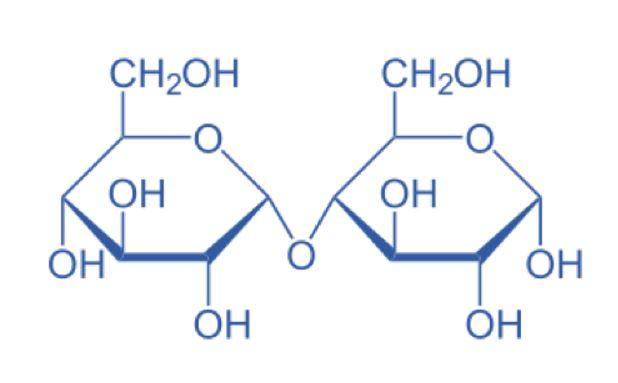
Fructose –
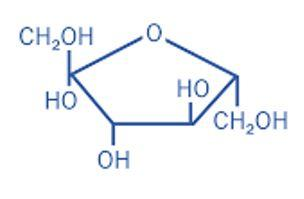
Galactose –
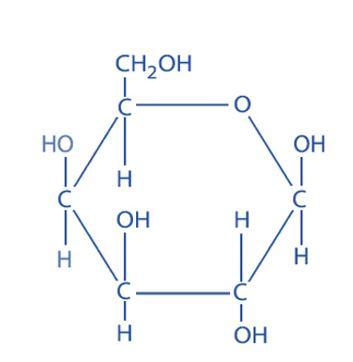
The anomeric carbon is the carbon where the ring forms between the hydroxyl carbon and the carbonyl carbon. Without this hydroxyl group, the ring cannot open and close and therefore not undergo mutarotation.
Glucose, fructose, maltose as well as galactose all have a free hydroxyl group and thus are known as reducing sugars. Hence, all of these will undergo mutarotation.
So, all options are correct.
Additional information: A hemiacetal forms when an aldehyde reacts with an alcohol. When glucose is in cyclic form, we can see hemiacetal linkage but it is not seen in a straight chain of glucose. Under acidic conditions the hemiacetal form of glucose can react with other alcohols to give acetals known as glycosides. These are widely distributed in nature.
Non reducing sugars such as sucrose cannot undergo mutarotation. This is because the glycosidic bond that forms between glucose and fructose in sucrose, eliminates the availability of hydroxyl groups.
Note: To remember it in an easy way, we can keep in mind that compounds which are cyclic and have a free hydroxyl group can only undergo mutarotation.
Complete step by step answer:
Mutarotation is the change in optical rotation because of the change in equilibrium of the two anomers, when corresponding stereocenters interconvert.
First, let us take a look at the structures of all the above-mentioned options:
Glucose –

Maltose –

Fructose –

Galactose –

The anomeric carbon is the carbon where the ring forms between the hydroxyl carbon and the carbonyl carbon. Without this hydroxyl group, the ring cannot open and close and therefore not undergo mutarotation.
Glucose, fructose, maltose as well as galactose all have a free hydroxyl group and thus are known as reducing sugars. Hence, all of these will undergo mutarotation.
So, all options are correct.
Additional information: A hemiacetal forms when an aldehyde reacts with an alcohol. When glucose is in cyclic form, we can see hemiacetal linkage but it is not seen in a straight chain of glucose. Under acidic conditions the hemiacetal form of glucose can react with other alcohols to give acetals known as glycosides. These are widely distributed in nature.
Non reducing sugars such as sucrose cannot undergo mutarotation. This is because the glycosidic bond that forms between glucose and fructose in sucrose, eliminates the availability of hydroxyl groups.
Note: To remember it in an easy way, we can keep in mind that compounds which are cyclic and have a free hydroxyl group can only undergo mutarotation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




