
Which of the following compounds will show geometrical isomerism.
A. 2-butene
B. Propene
C. 1-phenylpropene
D. 2-methyl-2-butene
Answer
578.1k+ views
Hint: Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers.
Complete step by step answer:
The word isomer is derived from the Greek words iso and mer which refers to the equal parts. There are two primary types of isomerism known as structural isomerism and stereoisomerism.
Structural isomerism is commonly defined as constitutional isomerism in which the functional groups and the atoms in the molecules are linked in different manners and stereoisomerism is the type of isomerism arises in compounds which have the same chemical formula but different orientations of the atoms belonging to the molecule in three-dimensional space. Geometric isomers is the type of stereoisomerism it can also be known by the cis-trans isomers and this isomers have different spatial arrangements of atoms in three-dimensional space.
To see which one will give geometrical isomer first write the structure of every option
A. 2-butene
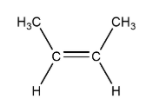
This can show cis and trans isomer so we can say that it shows geometrical isomerism
B. Propene
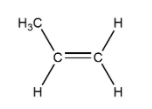
Cis and trans not possible
C. 1-phenylpropene
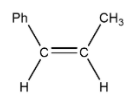
Cis and trans not possible
D. 2-methyl-2-butene
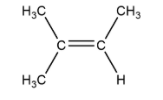
Cis and trans not possible
Thus, we can say that option A is the correct answer.
Note: Cis isomers are molecules with the same connectivity of atoms they have similar side groups placed on the same side of a double bond while Trans isomers have molecules with similar side groups placed on opposite sides of a double bond.
Complete step by step answer:
The word isomer is derived from the Greek words iso and mer which refers to the equal parts. There are two primary types of isomerism known as structural isomerism and stereoisomerism.
Structural isomerism is commonly defined as constitutional isomerism in which the functional groups and the atoms in the molecules are linked in different manners and stereoisomerism is the type of isomerism arises in compounds which have the same chemical formula but different orientations of the atoms belonging to the molecule in three-dimensional space. Geometric isomers is the type of stereoisomerism it can also be known by the cis-trans isomers and this isomers have different spatial arrangements of atoms in three-dimensional space.
To see which one will give geometrical isomer first write the structure of every option
A. 2-butene

This can show cis and trans isomer so we can say that it shows geometrical isomerism
B. Propene

Cis and trans not possible
C. 1-phenylpropene

Cis and trans not possible
D. 2-methyl-2-butene

Cis and trans not possible
Thus, we can say that option A is the correct answer.
Note: Cis isomers are molecules with the same connectivity of atoms they have similar side groups placed on the same side of a double bond while Trans isomers have molecules with similar side groups placed on opposite sides of a double bond.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




