
Which of the following compounds show haloform reaction and racemization in $ O{D^ - }|{D_2}O $ .
A. $ C{H_3}C{H_2}OH $
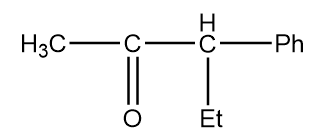
B.
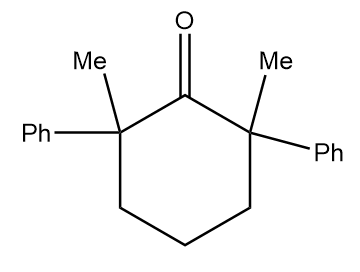
C.
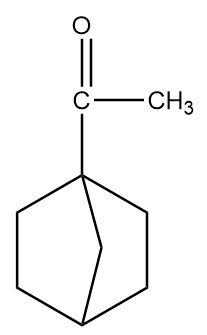
D.



Answer
513k+ views
Hint :Haloform reaction: It is the reaction in which methyl ketone reacts with dihalogen in the presence of dilute sodium hydroxide solution and formation of haloform i.e., $ CH{X_3} $ takes place along with the removal of sodium salt of acid.
Complete Step By Step Answer:
The major factor for a compound to undergo haloform reaction is that a methyl group must be attached to the carbonyl group. Let’s test the haloform reaction for each given option.
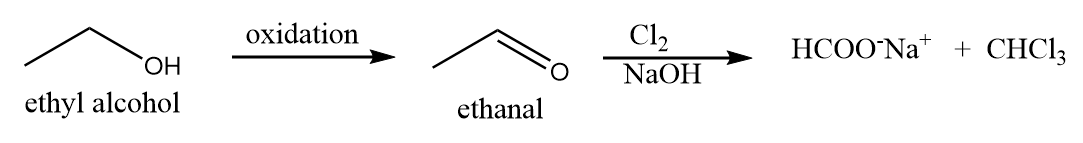
Structure given in option (A)- $ C{H_3}C{H_2}OH $ :
Ethyl alcohol when reacts with dihalogen in the presence of base, then it first gets oxidized under such conditions and forms ethanal which on halogenation reaction gives a yellow precipitate. Hence, ethyl alcohol gives a positive test for halogenation reaction. The reaction proceeds as follows:
Structure given in option (B)-
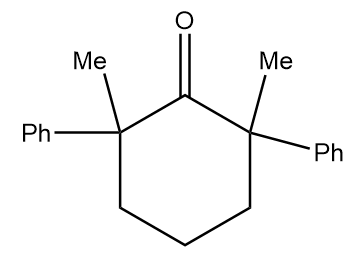
As in the given compound, no methyl group is present at the adjacent position to the carbonyl group, so it will not show a positive haloform test.
Structure given in option (C)-
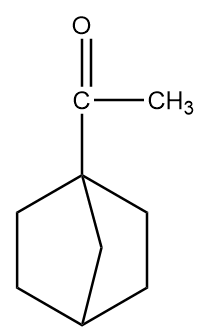
As in the given compound, one methyl group is present at the adjacent position to the carbonyl group, so it will show a positive haloform test.
Structure given in option (D)-
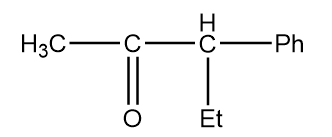
As in the given compound, one methyl group is present at the adjacent position to the carbonyl group, so it will show a positive haloform test.
Now, for the racemization reaction, when the acidic hydrogen is extracted by $ O{D^ - } $ then the carbon atom becomes $ s{p^2} $ hybridized i.e., the compound becomes planar and resonance stabilized. Therefore, the attack of electrophile is possible from the front as well as back side of the carbon atom and hence racemization of products will take place. This case is only possible in structure (D). The reaction is as follows:
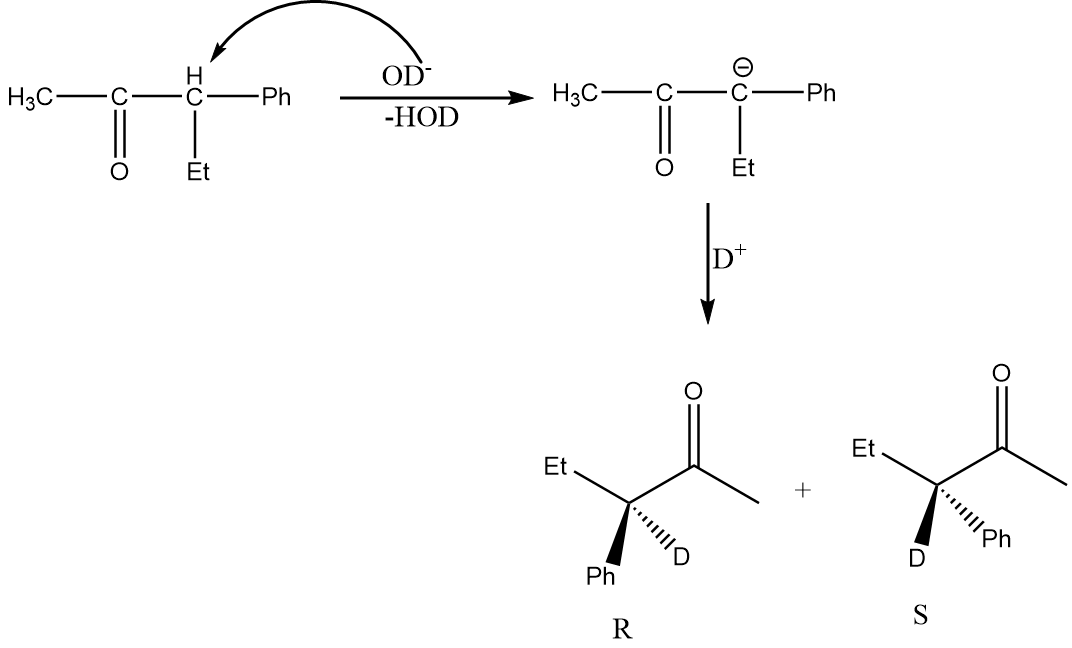
Hence, option (D) is the correct answer.
Note :
It is important to note that the Racemization process can be done in the molecule which consists of at least one chiral carbon. Therefore, although methyl groups consist of more acidic hydrogen, the racemization takes place at the carbon atom which is connected to four different groups and formation of $ 50\% $ R form and $ 50\% $ S form is observed which is known as a racemic mixture.
Complete Step By Step Answer:
The major factor for a compound to undergo haloform reaction is that a methyl group must be attached to the carbonyl group. Let’s test the haloform reaction for each given option.
Structure given in option (A)- $ C{H_3}C{H_2}OH $ :
Ethyl alcohol when reacts with dihalogen in the presence of base, then it first gets oxidized under such conditions and forms ethanal which on halogenation reaction gives a yellow precipitate. Hence, ethyl alcohol gives a positive test for halogenation reaction. The reaction proceeds as follows:

Structure given in option (B)-

As in the given compound, no methyl group is present at the adjacent position to the carbonyl group, so it will not show a positive haloform test.
Structure given in option (C)-

As in the given compound, one methyl group is present at the adjacent position to the carbonyl group, so it will show a positive haloform test.
Structure given in option (D)-

As in the given compound, one methyl group is present at the adjacent position to the carbonyl group, so it will show a positive haloform test.
Now, for the racemization reaction, when the acidic hydrogen is extracted by $ O{D^ - } $ then the carbon atom becomes $ s{p^2} $ hybridized i.e., the compound becomes planar and resonance stabilized. Therefore, the attack of electrophile is possible from the front as well as back side of the carbon atom and hence racemization of products will take place. This case is only possible in structure (D). The reaction is as follows:

Hence, option (D) is the correct answer.
Note :
It is important to note that the Racemization process can be done in the molecule which consists of at least one chiral carbon. Therefore, although methyl groups consist of more acidic hydrogen, the racemization takes place at the carbon atom which is connected to four different groups and formation of $ 50\% $ R form and $ 50\% $ S form is observed which is known as a racemic mixture.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




