
Which of the following compounds has three $1^\circ$, one $2^\circ$ and one $3^\circ$ carbon?
A.$C{H_3}{(C{H_2})_3}C{H_3}$
B.$C{H_3}CH(C{H_3})C{H_3}$
C.$C{H_3}{(C{H_2})_2}C{H_3}$
D.$C{H_3}CH(C{H_3})C{H_2}C{H_3}$
Answer
576.9k+ views
Hint:The carbon can be divided into primary $1^\circ$, secondary $2^\circ$, tertiary $3^\circ$and quaternary $4^\circ$ depending upon the number of carbon atoms attached to the single carbon atom by a single bond.
Complete step by step answer:
-When one carbon atom is attached directly to the carbon atom, then the carbon is said as primary carbon.
-When two carbon atoms are attached directly to the carbon atom, then the carbon is said as secondary carbon.
-When three carbon atoms are attached directly to the carbon atom, then the carbon is said as tertiary carbon.
-When four carbon atoms are attached directly to the carbon atom, then the carbon is said as quaternary carbon.
$C{H_3}{(C{H_2})_3}C{H_3}$
-The structure is shown below.
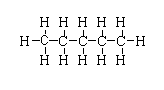
-In this compound, two primary carbon is present and three secondary carbon is present.
$C{H_3}CH(C{H_3})C{H_3}$
-The structure is shown below.
-In this compound, three primary carbon is present and one tertiary carbon is present.
$C{H_3}{(C{H_2})_2}C{H_3}$
-The structure is shown below.
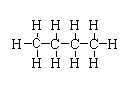
In this compound, two primary carbon is present and two secondary carbon is present.
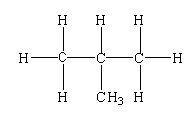
$C{H_3}CH(C{H_3})C{H_2}C{H_3}$
The structure is shown below.
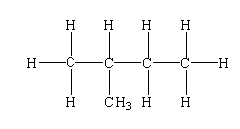
In this compound, one tertiary carbon, three primary carbon, and one secondary carbon are present.
Thus, the compound which has three $1^\circ$, one $2^\circ$ and one $3^\circ$ carbon is $C{H_3}CH(C{H_3})C{H_2}C{H_3}$.
Therefore, the correct option is D.
Note:
The carbon cannot exceed the quaternary carbon because to have five substituent carbon atoms, ten electrons are needed around the carbon atom which is not possible because in the valence orbital only eight electrons can be present according to the octet rule.
Complete step by step answer:
-When one carbon atom is attached directly to the carbon atom, then the carbon is said as primary carbon.
-When two carbon atoms are attached directly to the carbon atom, then the carbon is said as secondary carbon.
-When three carbon atoms are attached directly to the carbon atom, then the carbon is said as tertiary carbon.
-When four carbon atoms are attached directly to the carbon atom, then the carbon is said as quaternary carbon.
$C{H_3}{(C{H_2})_3}C{H_3}$
-The structure is shown below.

-In this compound, two primary carbon is present and three secondary carbon is present.
$C{H_3}CH(C{H_3})C{H_3}$
-The structure is shown below.

-In this compound, three primary carbon is present and one tertiary carbon is present.
$C{H_3}{(C{H_2})_2}C{H_3}$
-The structure is shown below.

In this compound, two primary carbon is present and two secondary carbon is present.
$C{H_3}CH(C{H_3})C{H_2}C{H_3}$
The structure is shown below.

In this compound, one tertiary carbon, three primary carbon, and one secondary carbon are present.
Thus, the compound which has three $1^\circ$, one $2^\circ$ and one $3^\circ$ carbon is $C{H_3}CH(C{H_3})C{H_2}C{H_3}$.
Therefore, the correct option is D.
Note:
The carbon cannot exceed the quaternary carbon because to have five substituent carbon atoms, ten electrons are needed around the carbon atom which is not possible because in the valence orbital only eight electrons can be present according to the octet rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




