
Which of the following carboxylic acid derivatives is most reactive towards nucleophilic substitution?
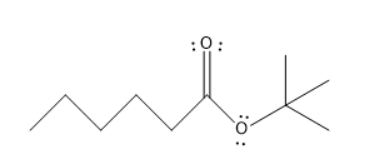
A. Tert-butyl hexanoate
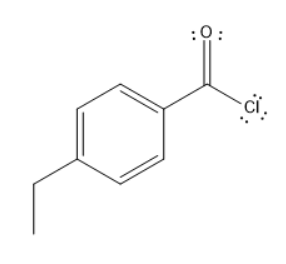
B. Para ethyl benzoyl chloride
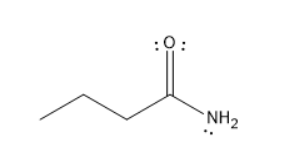
C. Butanamide
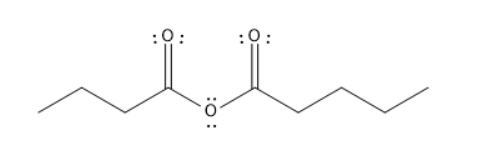
D. Butanoic pentanoic anhydride




Answer
489.9k+ views
Hint: Carboxylic acids are the chemical compounds that consist of a functional group \[ - COOH\] , the derivatives of carboxylic acids are amides, acyl chloride, acetic anhydride, and esters. In all these compounds acyl chloride is more reactive towards nucleophilic substitution reaction.
Complete answer:
Chemical compounds are classified based on the functional groups. Functional groups are the groups present in chemical compounds. Carboxylic acids are the chemical compounds that consist of a functional group \[ - COOH\] . There are different chemical compounds that can be called as derivatives of carboxylic acids. Some of them are acyl chlorides in the form of \[R - COCl\] , esters in the form of \[R - COO{R'}\] , acetic anhydride in the form of \[R - COOOR'\] where \[R\] and \[{R'}\] are different alkyl groups.
The given molecules are Tert-butyl hexanoate, Para ethyl benzoyl chloride, butanamide, and butanoic pentanoic anhydride.
In all these molecules, para ethyl benzoyl chloride has acyl group at the para position of ethyl group on benzene ring, which is a better leaving group. Thus, chlorine can easily leave the molecule by facilitating the nucleophilic substitution.
Note:
Nucleophilic substitution reaction means the replacement of one molecule or group which is nucleophile by another nucleophile. In para ethyl benzoyl chloride, the chlorine is an excellent leaving group. Better is the leaving group, the nucleophilic substitution will take more easily. The anhydride, ester, and amide are also good leaving groups but not as chlorine in acyl chloride.
Complete answer:
Chemical compounds are classified based on the functional groups. Functional groups are the groups present in chemical compounds. Carboxylic acids are the chemical compounds that consist of a functional group \[ - COOH\] . There are different chemical compounds that can be called as derivatives of carboxylic acids. Some of them are acyl chlorides in the form of \[R - COCl\] , esters in the form of \[R - COO{R'}\] , acetic anhydride in the form of \[R - COOOR'\] where \[R\] and \[{R'}\] are different alkyl groups.
The given molecules are Tert-butyl hexanoate, Para ethyl benzoyl chloride, butanamide, and butanoic pentanoic anhydride.
In all these molecules, para ethyl benzoyl chloride has acyl group at the para position of ethyl group on benzene ring, which is a better leaving group. Thus, chlorine can easily leave the molecule by facilitating the nucleophilic substitution.
Note:
Nucleophilic substitution reaction means the replacement of one molecule or group which is nucleophile by another nucleophile. In para ethyl benzoyl chloride, the chlorine is an excellent leaving group. Better is the leaving group, the nucleophilic substitution will take more easily. The anhydride, ester, and amide are also good leaving groups but not as chlorine in acyl chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




