
Which of the following are isomers of 1-pentyne?
(A) Penta-1,3-diene
(B) 2-methyl-1,3-butadiene
(C) Penta-1,2-diene
(D) All of the above.
Answer
582.6k+ views
Hint: When two or more compounds have the same molecular formula but a different arrangement of atoms in the molecule and have different properties from each other are known as isomers. Two main forms of isomerism are structural or constitutional isomerism in which bonds between the atoms are different.
Complete step by step answer:
There are three types of compound; alkane, alkene and alkyne. 1-pentyne is from the group of alkynes in which single and triple bonds are present. 1-pentyne consists a 5 carbon atoms in which triple bond is present in between the first and second carbon atom as:
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C\equiv CH\]
Isomers compounds are those compounds which have the same molecular formula but different arrangement of atoms and contain different properties. Molecular formula for 1-pentyne is \[{{C}_{5}}{{H}_{8}}\].
Penta-1,3-diene: Ene stands for alkene i.e. presence of double bond and single bond. It consist 5 carbon atoms and double bond is present at 1st and 3rd position which can be shown as:
\[C{{H}_{3}}-CH=CH-CH=C{{H}_{2}}\]
Molecular formula = \[{{C}_{5}}{{H}_{8}}\].
2-methyl-1,3-butadiene: In this compound double bond is present at 1st and 3rd position of 4-membered long chain called butane and on 2nd carbon atom of butane methyl group is attached which can be shown as:
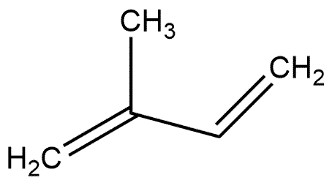
Molecular formula = \[{{C}_{5}}{{H}_{8}}\].
Penta-1,2-diene: Penta-1,2-diene consists two double bond present at 1st and 2nd position having 5 carbon atoms shown as:
\[C{{H}_{3}}-C{{H}_{2}}-CH=C=C{{H}_{2}}\]
Molecular formula = \[{{C}_{5}}{{H}_{8}}\]
In all the above cases the molecular formula remains the same, but the arrangement of atoms is different so these are all isomers of 1-pentyne.
Thus, the correct answer is d (All of the above)
Note: In case of pentane, pentene and pentene all these compounds have only 3 isomers but the number of isomers also increases with increase in the number of carbons in any atom. Isomers further be divided into structural or geometrical isomers. Structural isomers have the same number of atoms of each element because they have the same molecular formula, but the atoms are connected in logically distinct ways.
Complete step by step answer:
There are three types of compound; alkane, alkene and alkyne. 1-pentyne is from the group of alkynes in which single and triple bonds are present. 1-pentyne consists a 5 carbon atoms in which triple bond is present in between the first and second carbon atom as:
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C\equiv CH\]
Isomers compounds are those compounds which have the same molecular formula but different arrangement of atoms and contain different properties. Molecular formula for 1-pentyne is \[{{C}_{5}}{{H}_{8}}\].
Penta-1,3-diene: Ene stands for alkene i.e. presence of double bond and single bond. It consist 5 carbon atoms and double bond is present at 1st and 3rd position which can be shown as:
\[C{{H}_{3}}-CH=CH-CH=C{{H}_{2}}\]
Molecular formula = \[{{C}_{5}}{{H}_{8}}\].
2-methyl-1,3-butadiene: In this compound double bond is present at 1st and 3rd position of 4-membered long chain called butane and on 2nd carbon atom of butane methyl group is attached which can be shown as:

Molecular formula = \[{{C}_{5}}{{H}_{8}}\].
Penta-1,2-diene: Penta-1,2-diene consists two double bond present at 1st and 2nd position having 5 carbon atoms shown as:
\[C{{H}_{3}}-C{{H}_{2}}-CH=C=C{{H}_{2}}\]
Molecular formula = \[{{C}_{5}}{{H}_{8}}\]
In all the above cases the molecular formula remains the same, but the arrangement of atoms is different so these are all isomers of 1-pentyne.
Thus, the correct answer is d (All of the above)
Note: In case of pentane, pentene and pentene all these compounds have only 3 isomers but the number of isomers also increases with increase in the number of carbons in any atom. Isomers further be divided into structural or geometrical isomers. Structural isomers have the same number of atoms of each element because they have the same molecular formula, but the atoms are connected in logically distinct ways.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




