
Which of the above is a pair of geometrical isomers ?

A. I and II
B. I and III
C. II and IV
D. III and IV

Answer
590.7k+ views
Hint: For geometrical isomerism to be identified the bonding connectivity is verified. Those molecules having the same bonding connectivity can be geometrical isomers.
Complete answer:
-Stereoisomers are defined as the molecules having the same bonding connectivity and different molecular configuration.
-Stereoisomers can be of two types; Optical isomers or Geometrical Isomer
-Let us discuss geometrical isomers.
Geometrical Isomer is the isomer where the groups differ in arrangement along the double bonds, rings or other rigid structure.
for example;
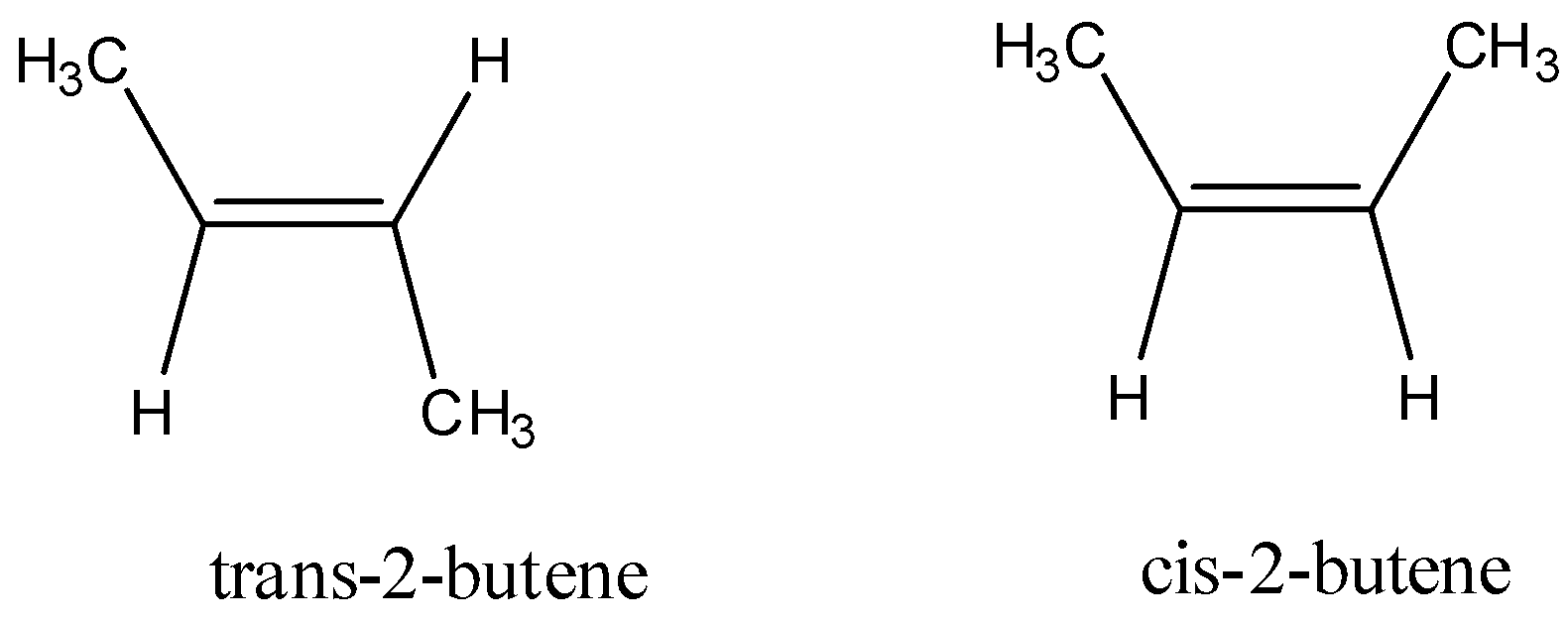
-Here along the double bond there are two methyl groups and two hydrogen, but their alignment along the double bond is different from each other. As we can see in trans-2-butane methyl groups are in opposite to each other and in case of cis-2-butane the methyl groups are in the same side of the double bond .
-Here, in this structure there is a double bond, therefore the structure is rigid, thus rotation along the double bond is not possible. In such cases only geometrical isomers can occur.
-Since geometrical isomers are stereoisomers too, thus geometrical isomers will also have the same bonding connectivity and different spatial arrangements of substituents.
-In Molecule II and IV they have same bonding connectivity i.e. Cl and H are connected to a particular carbon and on the adjacent carbon there are Br and $C{{H}_{3}}$, but they are connected in different arrangements.
Therefore, II and IV are geometrical Isomers.
Option C is correct.
Note:
To identify the geometrical isomerism we need to restrict the rotation of the carbon carbon double bond. And the main criteria of having geometrical isomers is to have different groups attached to a carbon atom.
Complete answer:
-Stereoisomers are defined as the molecules having the same bonding connectivity and different molecular configuration.
-Stereoisomers can be of two types; Optical isomers or Geometrical Isomer
-Let us discuss geometrical isomers.
Geometrical Isomer is the isomer where the groups differ in arrangement along the double bonds, rings or other rigid structure.
for example;
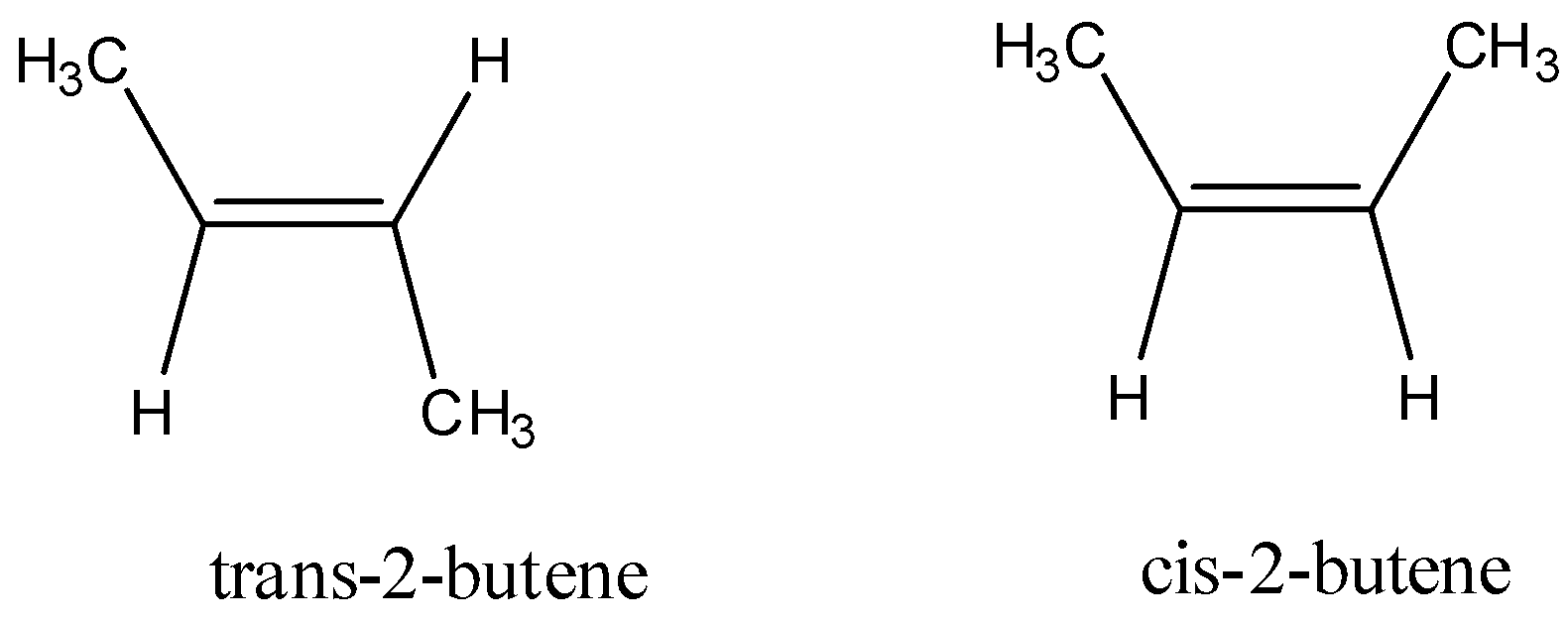
-Here along the double bond there are two methyl groups and two hydrogen, but their alignment along the double bond is different from each other. As we can see in trans-2-butane methyl groups are in opposite to each other and in case of cis-2-butane the methyl groups are in the same side of the double bond .
-Here, in this structure there is a double bond, therefore the structure is rigid, thus rotation along the double bond is not possible. In such cases only geometrical isomers can occur.
-Since geometrical isomers are stereoisomers too, thus geometrical isomers will also have the same bonding connectivity and different spatial arrangements of substituents.
-In Molecule II and IV they have same bonding connectivity i.e. Cl and H are connected to a particular carbon and on the adjacent carbon there are Br and $C{{H}_{3}}$, but they are connected in different arrangements.
Therefore, II and IV are geometrical Isomers.
Option C is correct.
Note:
To identify the geometrical isomerism we need to restrict the rotation of the carbon carbon double bond. And the main criteria of having geometrical isomers is to have different groups attached to a carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




