
Which is the major product formed in the following reaction?
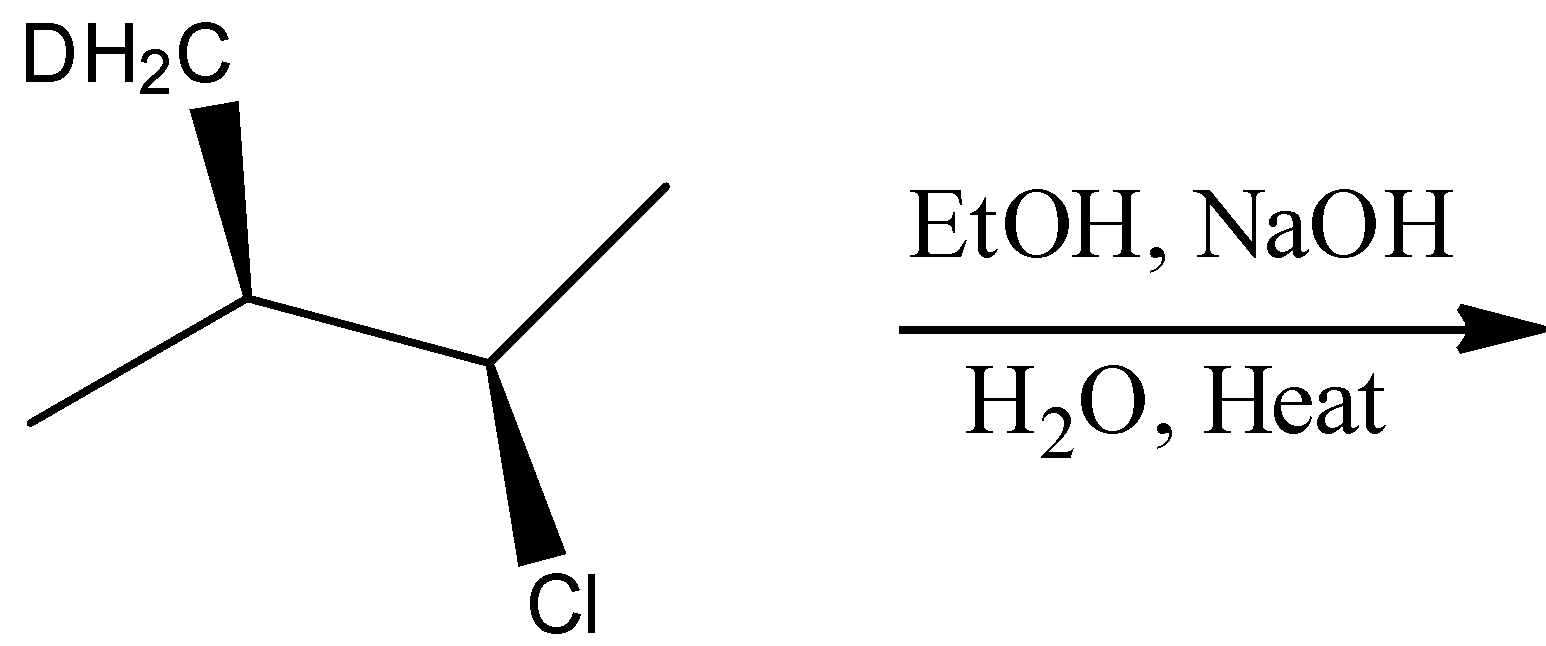
(A)
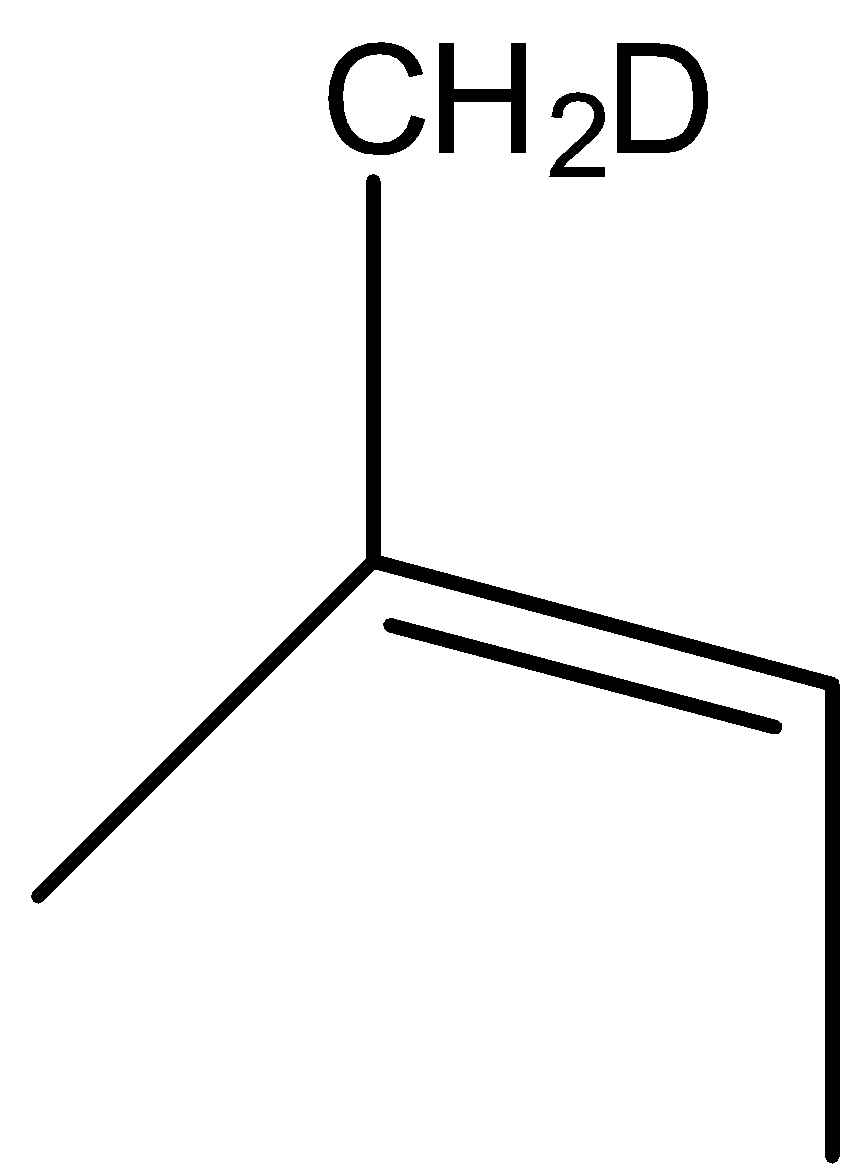
(B)
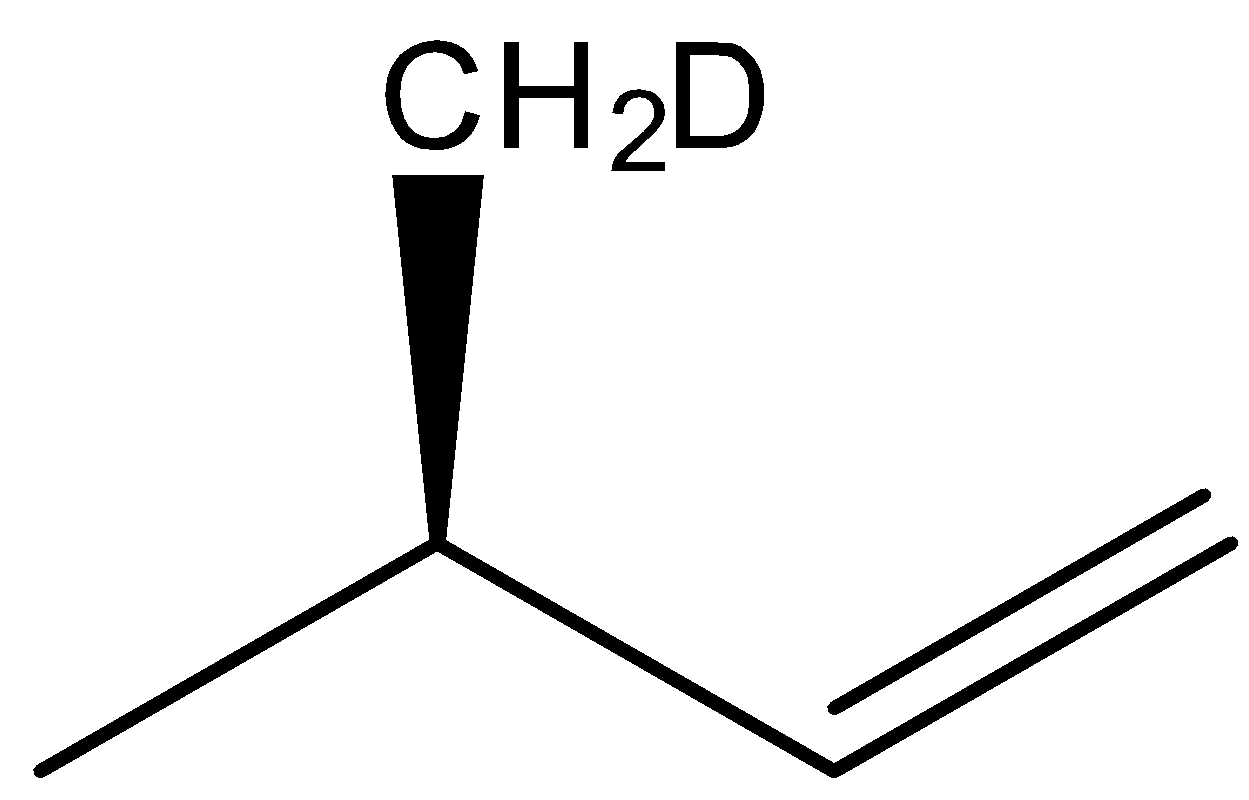
(C)
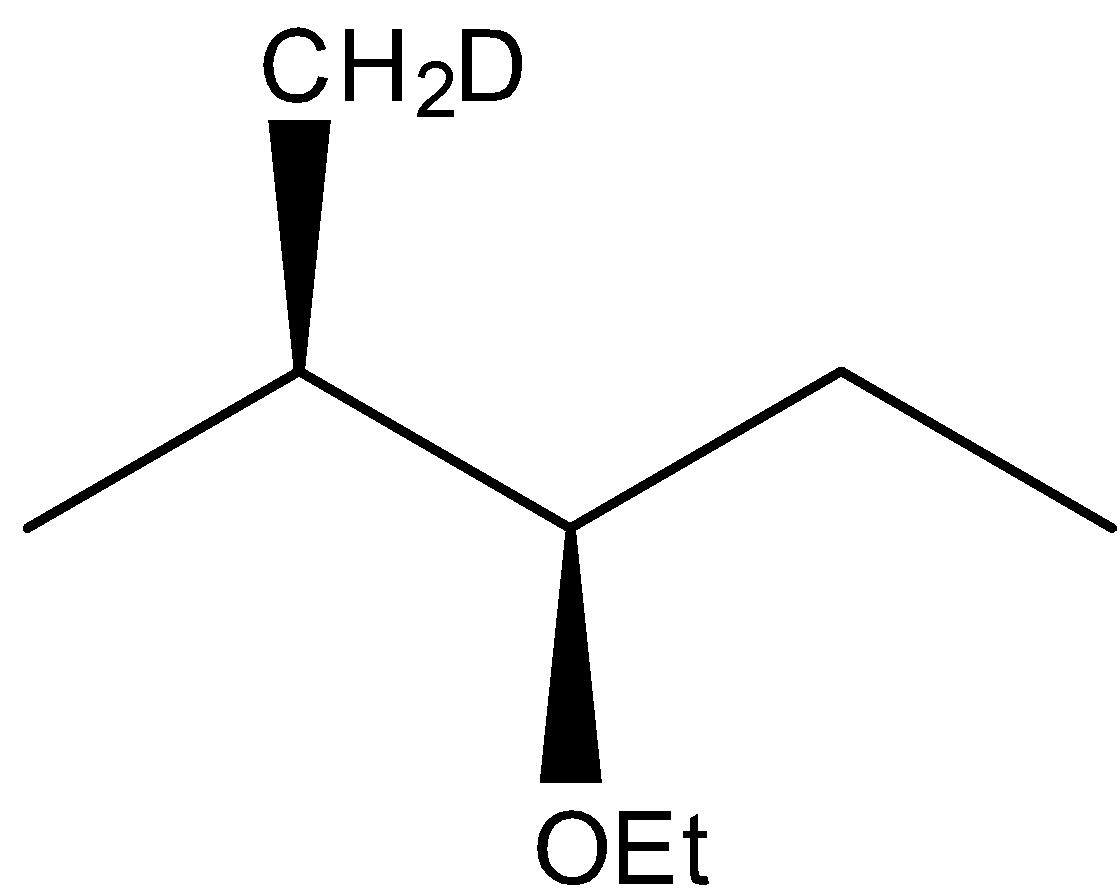
(D)
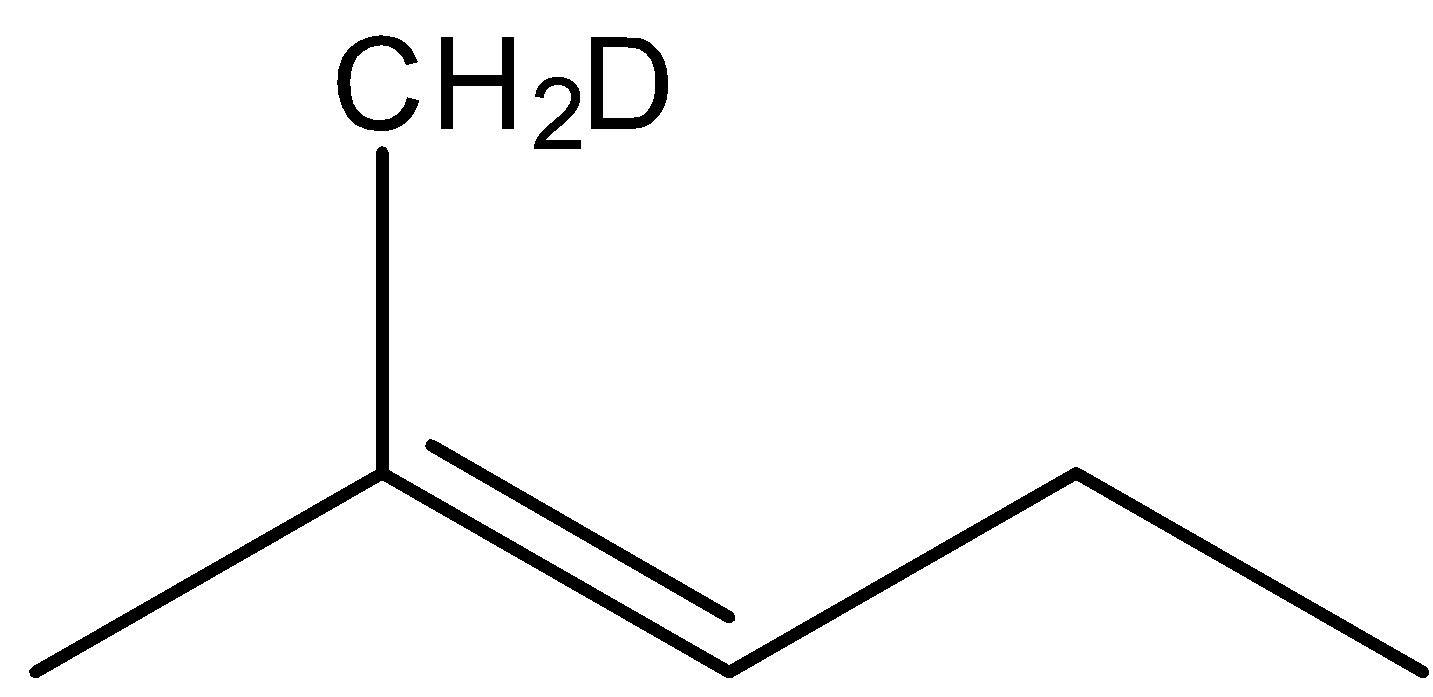





Answer
560.4k+ views
Hint: Weaker base $\text{(}{{\text{H}}_{\text{2}}}\text{O,halide)}$ leads to more substitution reaction, while in the presence of strong base $\text{(O}{{\text{H}}^{-}}\text{,R}{{\text{O}}^{-}}\text{,}{{\text{H}}_{\text{2}}}\text{N)}$ elimination reaction takes place.
- Sterically hindered (branch primary, secondary and tertiary) haloalkane mainly gives elimination reaction, while sterically unhindered (primary branched) haloalkane gives substitution reaction.
Sterically unhindered $\text{(O}{{\text{H}}^{\text{-}}}\text{,C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{\text{-}}}\text{,C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{-}}}\text{)}$ nucleophile leads to more substitution reaction, while sterically hindered nucleophile gives elimination reaction.
Complete step by step answer:
- The relative proportion of product in a reaction mainly depends on the basicity of the nucleophile, hindrance in the haloalkane, and steric hindrance around the nucleophilic atom.
- In this given reaction a branched chain primary haloalkane is reacting with sterically unhindered nucleophile ethyl alcohol in the presence of a strong base. So in the presence of a strong base a ${{2}^{\circ }}$ halide gives elimination reaction as a major product and substitution reaction as a minor product.
- Hence in this reaction bimolecular elimination and substitution reaction takes place, this reaction is represented by following equation-
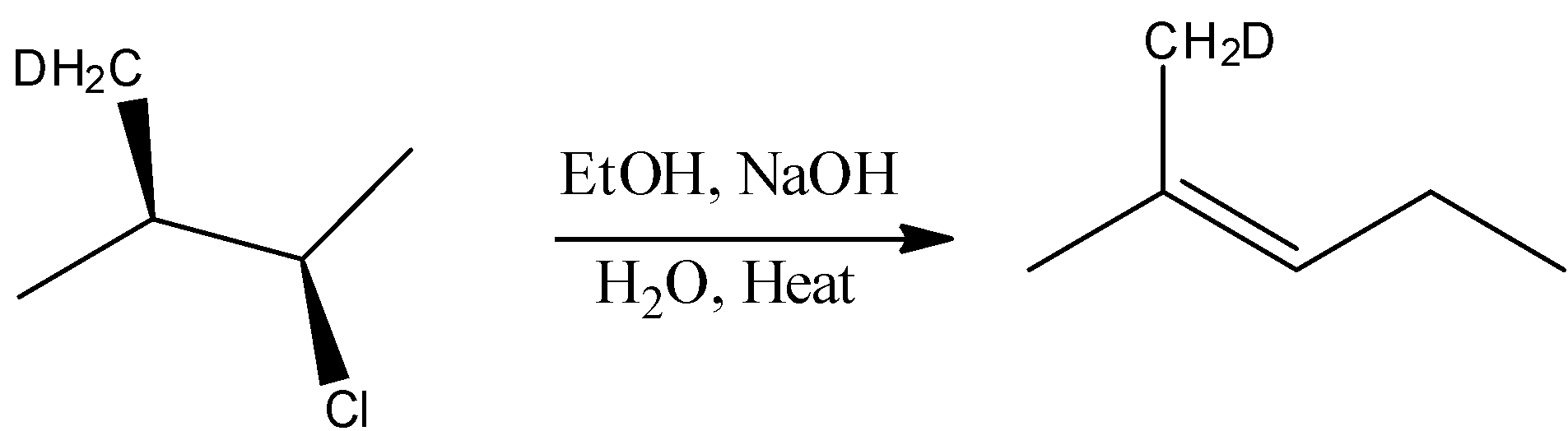
The correct answer is option “D” .
Note: In the presence of $\text{alc}\text{.NaOH}$ and heating, elimination reaction occurs, as a result of 1-2 elimination formation of double bond take place. If more than one product is possible, the more substituted alkene or more stable alkene will be the major product (according to Saytzeff rule).
- ${{1}^{\circ }}$ and ${{2}^{\circ }}$ Halide mainly gives bimolecular elimination and substitution reaction in the presence of a strong base. Reactivity order alkyl halide toward bimolecular elimination reaction is ${{3}^{\circ }}>\,{{2}^{\circ }}>\,{{1}^{\circ }}$
- A polar or protic solvent favours the elimination reaction, while a non-polar or aprotic solvent favours the substitution reaction.
- Sterically hindered (branch primary, secondary and tertiary) haloalkane mainly gives elimination reaction, while sterically unhindered (primary branched) haloalkane gives substitution reaction.
Sterically unhindered $\text{(O}{{\text{H}}^{\text{-}}}\text{,C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{\text{-}}}\text{,C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}{{\text{O}}^{\text{-}}}\text{)}$ nucleophile leads to more substitution reaction, while sterically hindered nucleophile gives elimination reaction.
Complete step by step answer:
- The relative proportion of product in a reaction mainly depends on the basicity of the nucleophile, hindrance in the haloalkane, and steric hindrance around the nucleophilic atom.
- In this given reaction a branched chain primary haloalkane is reacting with sterically unhindered nucleophile ethyl alcohol in the presence of a strong base. So in the presence of a strong base a ${{2}^{\circ }}$ halide gives elimination reaction as a major product and substitution reaction as a minor product.
- Hence in this reaction bimolecular elimination and substitution reaction takes place, this reaction is represented by following equation-
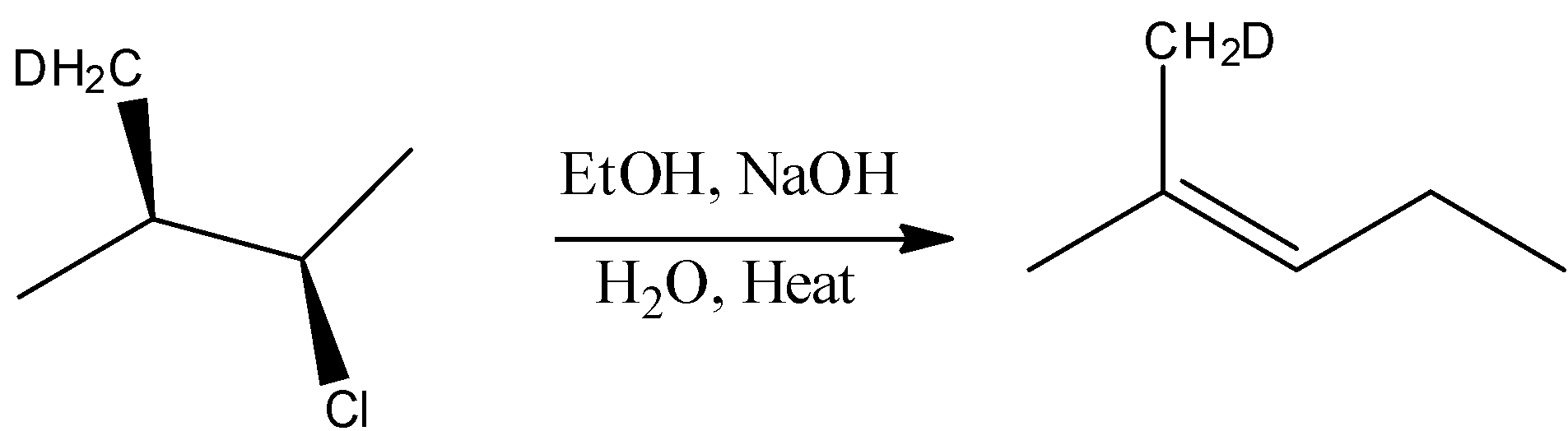
The correct answer is option “D” .
Note: In the presence of $\text{alc}\text{.NaOH}$ and heating, elimination reaction occurs, as a result of 1-2 elimination formation of double bond take place. If more than one product is possible, the more substituted alkene or more stable alkene will be the major product (according to Saytzeff rule).
- ${{1}^{\circ }}$ and ${{2}^{\circ }}$ Halide mainly gives bimolecular elimination and substitution reaction in the presence of a strong base. Reactivity order alkyl halide toward bimolecular elimination reaction is ${{3}^{\circ }}>\,{{2}^{\circ }}>\,{{1}^{\circ }}$
- A polar or protic solvent favours the elimination reaction, while a non-polar or aprotic solvent favours the substitution reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




