
Which compound will have the highest boiling point?
A.$C{H_4}$
B.$C{H_3}OH$
C.${C_2}{H_5}OH$
D.$HCHO$
Answer
564.6k+ views
Hint: The molecules with stronger the intermolecular forces of attraction show higher boiling points.The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
Complete step by step answer:
The given options contains methane ($C{H_4}$), methanol ($C{H_3}OH$), ethanol (${C_2}{H_5}OH$) and formaldehyde ($HCHO$). Among the following, the one which contains the strongest intermolecular forces will have higher boiling point. Because, greater the attraction, more energy will be needed to break the bonds and hence higher will be the boiling point.
The presence of hydrogen bonds play an important role in determining the boiling point of different substances. Hydrogen bond is a special type of dipole-dipole interaction. It is the attractive force operating between hydrogen atom covalently bonded to a high electronegative atom and another high electronegative atom. Molecules with hydrogen bonds possess high boiling point. The hydrogen bonds make molecules stickier.
Among the following options, hydrogen bond is present in methanol and ethanol. Because in both methanol and ethanol hydrogen is bonded to oxygen, a highly electronegative element. Thus this hydrogen can form hydrogen bonds with oxygen atoms of another molecule. Hydrogen bonds in methanol and ethanol are shown in the figure.
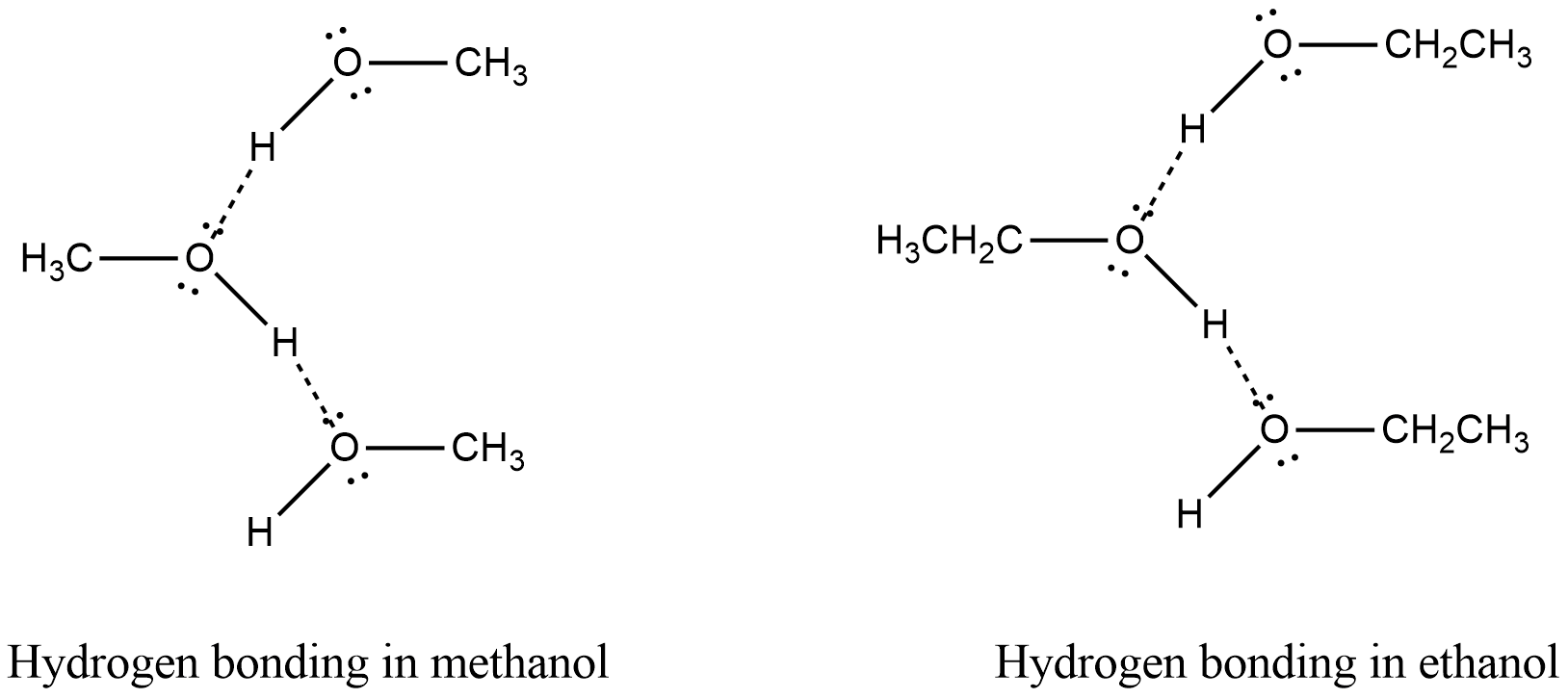
In methane and formaldehyde, no hydrogen bond is present. Hence they have a low boiling point.
Ethanol possesses a higher boiling point than methanol. As the number of carbons increases, boiling point of alcohol increases. For alcohols, boiling point depends on van-der Waals dispersion forces also. This force increases as the length of the hydrocarbon chain increases. Hence ethanol possesses a higher boiling point than methanol.
And hence the correct option is C.
Note:
-Alcohols possess higher boiling point than alkane, alkyl halides, aldehydes and ketone of comparable molecular mass because of the presence of intermolecular hydrogen bonds in alcohols.
-In the given options, formaldehyde also contains an electronegative atom (oxygen) and hydrogen. But its boiling point is low, because hydrogen is not directly bonded to oxygen. Hence it does not have a strong hydrogen bond.
Complete step by step answer:
The given options contains methane ($C{H_4}$), methanol ($C{H_3}OH$), ethanol (${C_2}{H_5}OH$) and formaldehyde ($HCHO$). Among the following, the one which contains the strongest intermolecular forces will have higher boiling point. Because, greater the attraction, more energy will be needed to break the bonds and hence higher will be the boiling point.
The presence of hydrogen bonds play an important role in determining the boiling point of different substances. Hydrogen bond is a special type of dipole-dipole interaction. It is the attractive force operating between hydrogen atom covalently bonded to a high electronegative atom and another high electronegative atom. Molecules with hydrogen bonds possess high boiling point. The hydrogen bonds make molecules stickier.
Among the following options, hydrogen bond is present in methanol and ethanol. Because in both methanol and ethanol hydrogen is bonded to oxygen, a highly electronegative element. Thus this hydrogen can form hydrogen bonds with oxygen atoms of another molecule. Hydrogen bonds in methanol and ethanol are shown in the figure.

In methane and formaldehyde, no hydrogen bond is present. Hence they have a low boiling point.
Ethanol possesses a higher boiling point than methanol. As the number of carbons increases, boiling point of alcohol increases. For alcohols, boiling point depends on van-der Waals dispersion forces also. This force increases as the length of the hydrocarbon chain increases. Hence ethanol possesses a higher boiling point than methanol.
And hence the correct option is C.
Note:
-Alcohols possess higher boiling point than alkane, alkyl halides, aldehydes and ketone of comparable molecular mass because of the presence of intermolecular hydrogen bonds in alcohols.
-In the given options, formaldehyde also contains an electronegative atom (oxygen) and hydrogen. But its boiling point is low, because hydrogen is not directly bonded to oxygen. Hence it does not have a strong hydrogen bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




