
Which among the following is an inorganic compound?
A) Protein
B) Marble
C) Waxes
D) Sugar
Answer
578.4k+ views
Hint: Identify the compounds that contain carbon-hydrogen bonds, Classify these compounds as organic compounds. Identify the compounds that do not contain carbon-hydrogen bonds, Classify these compounds as inorganic compounds.
Complete answer:
You can classify the compounds in two types: organic compounds and inorganic compounds.
An organic compound contains a carbon-hydrogen bond. The examples of organic compounds include methane, ethane, ethylene acetylene etc.
Given below is the structure of methane.
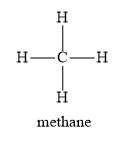
You will observe that one methane molecule contains four carbon-hydrogen sigma bonds. Hence, methane is an organic compound.
Protein, waxes, and sugar contains carbon-hydrogen bonds. Hence, they are organic compounds.
On the other hand, an inorganic compound is a compound that does not contain a carbon-hydrogen bond. Examples of inorganic compounds include hydrochloric acid, sulphuric acid, nitric acid, sodium hydroxide, potassium carbonate etc.
Marble contains calcium carbonate. Its chemical formula is \[{\text{CaC}}{{\text{O}}_3}\] . In marble, no carbon-hydrogen bond is present.
Thus, marble is an inorganic compound.
Given below is the structure of calcium carbonate (marble).
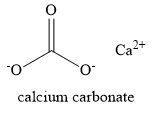
In the above structure, you cannot find a carbon-hydrogen bond. Thus, you can classify marble as an inorganic compound.
Hence, the correct option is the option (B).
Note: Proteins are polypeptides made from several amino acids and contain peptide bonds (amide linkage). Sugars are carbohydrates (or hydrates of carbon). They can be further classified into monosaccharides, disaccharides, polysaccharides etc. Waxes contain higher alkanes and lipids.
Complete answer:
You can classify the compounds in two types: organic compounds and inorganic compounds.
An organic compound contains a carbon-hydrogen bond. The examples of organic compounds include methane, ethane, ethylene acetylene etc.
Given below is the structure of methane.

You will observe that one methane molecule contains four carbon-hydrogen sigma bonds. Hence, methane is an organic compound.
Protein, waxes, and sugar contains carbon-hydrogen bonds. Hence, they are organic compounds.
On the other hand, an inorganic compound is a compound that does not contain a carbon-hydrogen bond. Examples of inorganic compounds include hydrochloric acid, sulphuric acid, nitric acid, sodium hydroxide, potassium carbonate etc.
Marble contains calcium carbonate. Its chemical formula is \[{\text{CaC}}{{\text{O}}_3}\] . In marble, no carbon-hydrogen bond is present.
Thus, marble is an inorganic compound.
Given below is the structure of calcium carbonate (marble).

In the above structure, you cannot find a carbon-hydrogen bond. Thus, you can classify marble as an inorganic compound.
Hence, the correct option is the option (B).
Note: Proteins are polypeptides made from several amino acids and contain peptide bonds (amide linkage). Sugars are carbohydrates (or hydrates of carbon). They can be further classified into monosaccharides, disaccharides, polysaccharides etc. Waxes contain higher alkanes and lipids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




