
What is the Lewis structure of \[C{O_2}\]?
Answer
519.3k+ views
Hint: We have to know that the Lewis structure can be studied to get the molecular geometry for any molecule. We can easily get the number of electrons evolved in a molecule, its shape and how these numbers of electrons are arranged in a molecule. Valence electrons are the electrons that are present in the outermost shell of an atom.
Complete answer:
Before starting this question we must know what Lewis structure is? Lewis structure can be drawn by arranging the outermost electrons for atoms that are present in a molecule. The dots are drawn to get valence electrons for each atom involved. And when we draw a line to join the two atoms they represent a bond.
Now let’s see the molecule of \[C{O_2}\], it has one carbon atom and two oxygen atoms. The carbon atom is placed in the centre while the two oxygen atoms in the neighbor of the carbon atom or you can say at the terminals. Since Carbon is least electronegative thus placed at centre and both oxygen atoms share electrons to form bond between Carbon and oxygen.
We can draw the structure of carbon dioxide:
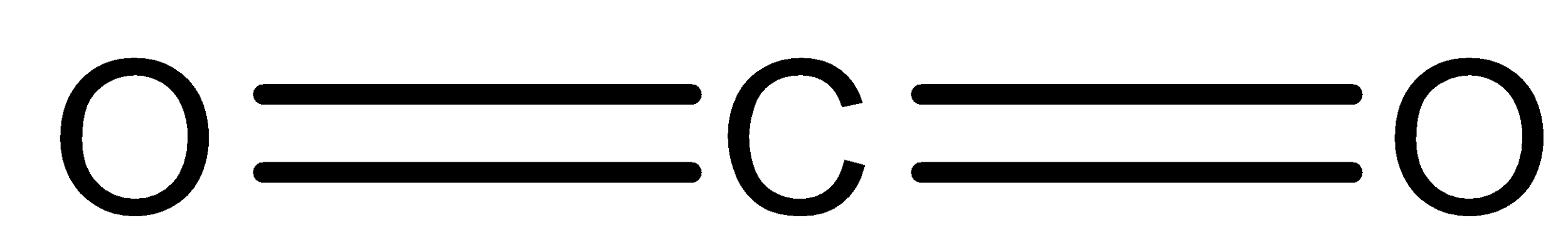
Lewis dot structure of carbon dioxide: Valence electron in carbon is \[4\] whereas Oxygen has \[6\] valence electrons; thus
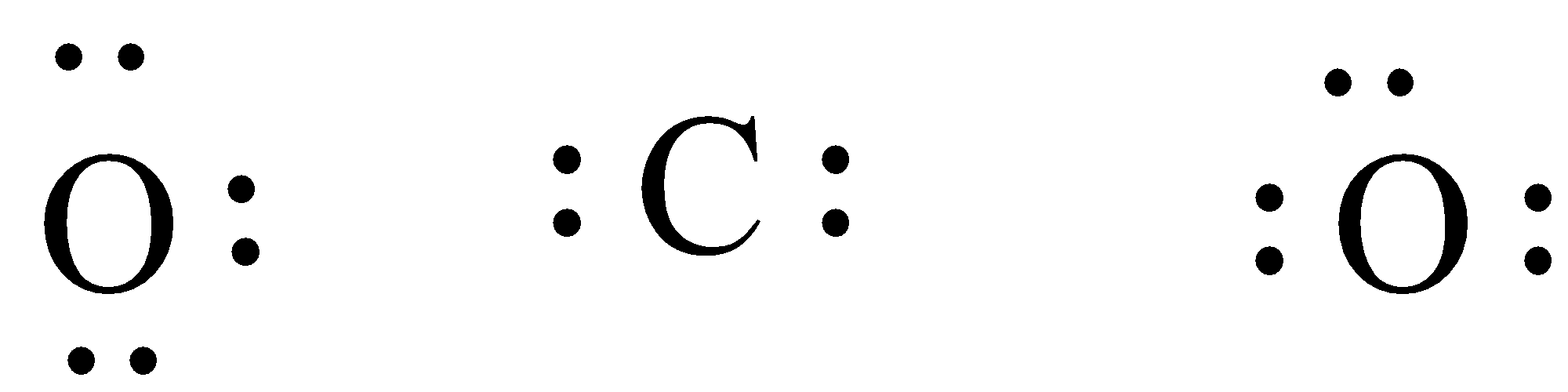
Note:
We must have to know that the total valence electrons of carbon dioxide can be calculated by adding the valence electrons of oxygen and carbon atoms. Since there are two oxygen atoms, the valence electron for oxygen is \[\left( {6 \times 2} \right)\] and valence electron for carbon is \[4\] thus the total valence electron will be \[16\] for carbon dioxide.
Complete answer:
Before starting this question we must know what Lewis structure is? Lewis structure can be drawn by arranging the outermost electrons for atoms that are present in a molecule. The dots are drawn to get valence electrons for each atom involved. And when we draw a line to join the two atoms they represent a bond.
Now let’s see the molecule of \[C{O_2}\], it has one carbon atom and two oxygen atoms. The carbon atom is placed in the centre while the two oxygen atoms in the neighbor of the carbon atom or you can say at the terminals. Since Carbon is least electronegative thus placed at centre and both oxygen atoms share electrons to form bond between Carbon and oxygen.
We can draw the structure of carbon dioxide:
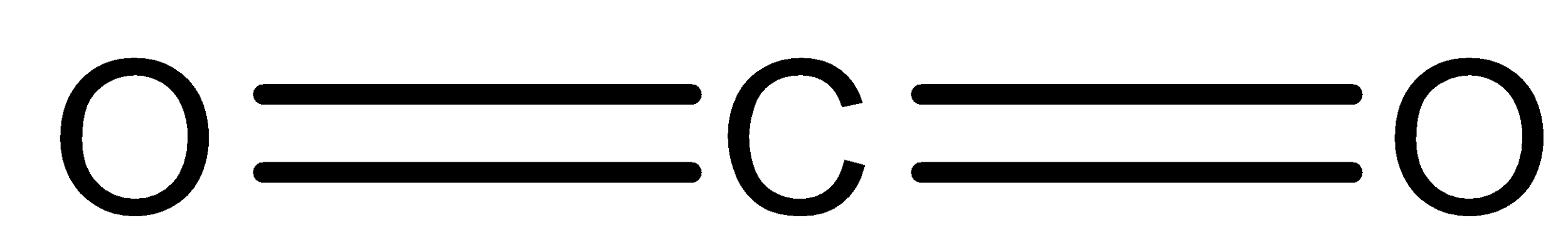
Lewis dot structure of carbon dioxide: Valence electron in carbon is \[4\] whereas Oxygen has \[6\] valence electrons; thus
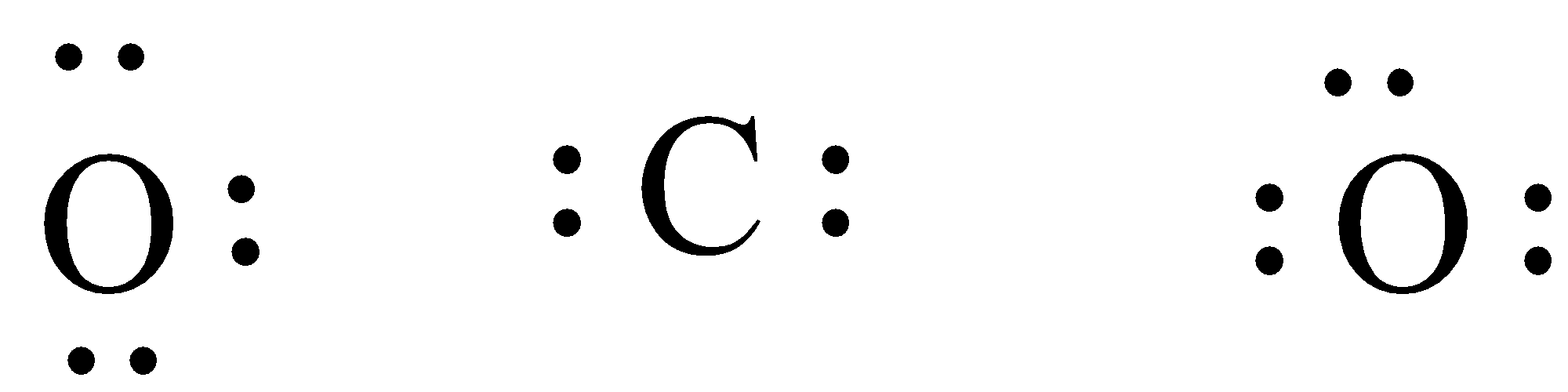
Note:
We must have to know that the total valence electrons of carbon dioxide can be calculated by adding the valence electrons of oxygen and carbon atoms. Since there are two oxygen atoms, the valence electron for oxygen is \[\left( {6 \times 2} \right)\] and valence electron for carbon is \[4\] thus the total valence electron will be \[16\] for carbon dioxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




