
What is the dipole moment of benzene?
Answer
513.3k+ views
Hint: Benzene is hydrocarbon, carbon and hydrogen have similar electronegativities. Therefore there is no shift in electron density. Dipole moment can be calculated by product of the magnitude of the charge and the distance between the centers of the positive and negative charges or dipole is the measure of polarity of the molecule.
Complete answer: \[ \bullet \]Dipole moment is denoted by \[\mu \], measured in Debye units which is denoted by 'D'. Where $1D = 3.33564 \times {10^{ - 30}}$ Cm. ( C is Coulomb and m is meter).
\[ \bullet \]
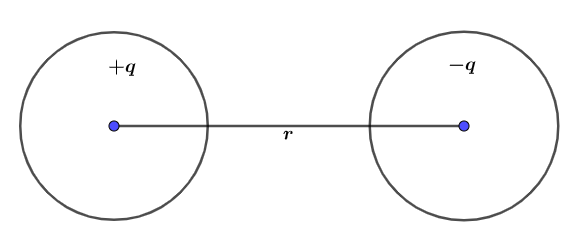
\[\mu = q.r\]
\[\mu \] is dipole moment, \[q\] is a separated charge and \[r\]is distance between them.
\[ \bullet \]Benzene \[{C_6}{H_6}\]
\[ \bullet \] Benzene is an organic compound, consisting of 6 carbon atoms joined in a planar ring with one hydrogen atom attached to each carbon. It contains only carbon and hydrogen. Therefore, it is a hydrocarbon.
\[ \bullet \] Molecules which have dipole moments are either asymmetrical or have different electronegativities.
\[ \bullet \] Dipole moment of benzene is zero. It's not exactly zero, but negligible.
\[ \bullet \] Benzene is hydrocarbon, carbon and hydrogen have similar electronegativities. Therefore there is no shift in electron density.
\[ \bullet \] Benzene is aromatic, due to resonance net charge displacement is almost zero . It has symmetrical planar, the $C-H$ bonds or the six dipole moments cancel out one another. Hence, the net dipole is zero and all the individual dipoles cancel out.
\[ \bullet \] A dipole moment arises anywhere there is a separation of charge. Therefore, they arise in ionic bonds as well as in covalent bonds.
And hence the dipole moment of Benzene is zero.
Note:
Make sure that you don't confuse benzene with its derivatives such as phenol, toluene and aniline. The bond dipole moment is a vector quantity as it has both magnitude and direction
Diagrams are necessary to solve such problems as it eases the question.
Complete answer: \[ \bullet \]Dipole moment is denoted by \[\mu \], measured in Debye units which is denoted by 'D'. Where $1D = 3.33564 \times {10^{ - 30}}$ Cm. ( C is Coulomb and m is meter).
\[ \bullet \]

\[\mu = q.r\]
\[\mu \] is dipole moment, \[q\] is a separated charge and \[r\]is distance between them.
\[ \bullet \]Benzene \[{C_6}{H_6}\]

\[ \bullet \] Benzene is an organic compound, consisting of 6 carbon atoms joined in a planar ring with one hydrogen atom attached to each carbon. It contains only carbon and hydrogen. Therefore, it is a hydrocarbon.
\[ \bullet \] Molecules which have dipole moments are either asymmetrical or have different electronegativities.
\[ \bullet \] Dipole moment of benzene is zero. It's not exactly zero, but negligible.
\[ \bullet \] Benzene is hydrocarbon, carbon and hydrogen have similar electronegativities. Therefore there is no shift in electron density.
\[ \bullet \] Benzene is aromatic, due to resonance net charge displacement is almost zero . It has symmetrical planar, the $C-H$ bonds or the six dipole moments cancel out one another. Hence, the net dipole is zero and all the individual dipoles cancel out.
\[ \bullet \] A dipole moment arises anywhere there is a separation of charge. Therefore, they arise in ionic bonds as well as in covalent bonds.
And hence the dipole moment of Benzene is zero.
Note:
Make sure that you don't confuse benzene with its derivatives such as phenol, toluene and aniline. The bond dipole moment is a vector quantity as it has both magnitude and direction
Diagrams are necessary to solve such problems as it eases the question.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




