
How many valence electrons does krypton have?
(A) 36
(B) 6
(C) 8
(D) 4
(E) 10
Answer
527.7k+ views
Hint:
As in this Question, we have to find the valence electrons. Firstly, Valence electron is an outer shell electron that is associated with an atom. It is located in the outermost shell of an atom; it is a negatively charged particle and it is transferred or shared with another atom. Let us take an example of Oxygen just to understand, as Oxygen has $6$ Valence electrons, two in the \[2s\] Subshell four in the \[2p\] subshell. So, the Configuration of Oxygen’s valence electrons is \[2{s^2}2{p^4}\]
Complete step by step answer:
As we know that Krypton is a chemical element & its symbol is Kr. Its atomic number is\[36\]. Talking about some main properties so it is a colorless, odorless, tasteless noble gas that occurs in the atmosphere and as in the question we have to calculate the valence electrons of Krypton since we discussed that Kr is a noble gas. It has a full valence shell or it also has an octet of \[8\]electrons. We can write the Electronic Configuration of Krypton as follows:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}\]
Therefore, the valence shell of krypton is \[4\] and hence there are \[8\] electrons in this valence shell.
Moreover, we discuss the easiest method of finding valence electrons. So, for neutral atoms, the number of valence electrons is equal to the atoms main group number. And the group number of the Non-Transition metal can be used to find the valence electrons in that element. The main group member for an element can be found from its column on the periodic table. The one place of the group number is the number of valence electrons in an atom of these elements.
For Example: Carbon is in group \[4\] & it has \[4\] valence electrons.
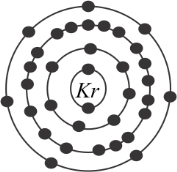
Hence Option C is the correct one. As Krypton has \[8\] valence electrons.
Note:
Krypton is used in some flash lamps which are used for high-speed photography. For Energy Saving fluorescent lights, it is used as filling of gas.
As in this Question, we have to find the valence electrons. Firstly, Valence electron is an outer shell electron that is associated with an atom. It is located in the outermost shell of an atom; it is a negatively charged particle and it is transferred or shared with another atom. Let us take an example of Oxygen just to understand, as Oxygen has $6$ Valence electrons, two in the \[2s\] Subshell four in the \[2p\] subshell. So, the Configuration of Oxygen’s valence electrons is \[2{s^2}2{p^4}\]
Complete step by step answer:
As we know that Krypton is a chemical element & its symbol is Kr. Its atomic number is\[36\]. Talking about some main properties so it is a colorless, odorless, tasteless noble gas that occurs in the atmosphere and as in the question we have to calculate the valence electrons of Krypton since we discussed that Kr is a noble gas. It has a full valence shell or it also has an octet of \[8\]electrons. We can write the Electronic Configuration of Krypton as follows:
\[1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^{10}}4{s^2}4{p^6}\]
Therefore, the valence shell of krypton is \[4\] and hence there are \[8\] electrons in this valence shell.
Moreover, we discuss the easiest method of finding valence electrons. So, for neutral atoms, the number of valence electrons is equal to the atoms main group number. And the group number of the Non-Transition metal can be used to find the valence electrons in that element. The main group member for an element can be found from its column on the periodic table. The one place of the group number is the number of valence electrons in an atom of these elements.
For Example: Carbon is in group \[4\] & it has \[4\] valence electrons.

Hence Option C is the correct one. As Krypton has \[8\] valence electrons.
Note:
Krypton is used in some flash lamps which are used for high-speed photography. For Energy Saving fluorescent lights, it is used as filling of gas.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




