
How many unpaired electrons in a ground state fluorine atom?
Answer
540.6k+ views
Hint :So write the number of unpaired electrons in the ground state firstly we have to write its electronic configuration. To write the electronic configuration we need to follow the Auf bau and Hund's rule. The auf bau principle states that the electrons need to occupy those orbitals first which have the lowest energy. The Hund's rule states that before the orbital gets doubly occupied, the orbital in the sub level should be singly occupied.
Complete Step By Step Answer:
So first to write the electronic configuration of fluorine we need to know its atomic number. The atomic number of fluorine is 9. It is in the period 2 and group 17. So as its atomic number is 9 so it has 9 electrons and 9 protons in it. So its electronic configuration will be:
$ 1{s^2}2{s^2}2{p^5} $
So as I said that by the Hund's rule if there are two or more orbitals then we need to fill each of the orbital by 1 electron equally then the process of pairing takes place. So the orbital filling of shell is represent as follows:
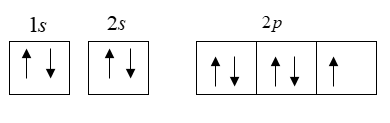
Here in the s subshell we have only one orbital but in p subshell we have three orbitals. So we are able to see that 1 electron which is present in the p subshell is unpaired whereas in other orbitals the electrons are paired.
So in the ground state of fluorine we have only 1 unpaired electron.
Note :
The order by which the energy of the orbital increases can be found by the rule called (n+l) rule. Here we need to sum the principal as well as the azimuthal quantum number. If the (n+l) value is lower than the orbitals of energy will be lower. The for filling the orbitals in 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Complete Step By Step Answer:
So first to write the electronic configuration of fluorine we need to know its atomic number. The atomic number of fluorine is 9. It is in the period 2 and group 17. So as its atomic number is 9 so it has 9 electrons and 9 protons in it. So its electronic configuration will be:
$ 1{s^2}2{s^2}2{p^5} $
So as I said that by the Hund's rule if there are two or more orbitals then we need to fill each of the orbital by 1 electron equally then the process of pairing takes place. So the orbital filling of shell is represent as follows:

Here in the s subshell we have only one orbital but in p subshell we have three orbitals. So we are able to see that 1 electron which is present in the p subshell is unpaired whereas in other orbitals the electrons are paired.
So in the ground state of fluorine we have only 1 unpaired electron.
Note :
The order by which the energy of the orbital increases can be found by the rule called (n+l) rule. Here we need to sum the principal as well as the azimuthal quantum number. If the (n+l) value is lower than the orbitals of energy will be lower. The for filling the orbitals in 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




