
Two different compounds have the formula \[{\text{Xe}}{{\text{F}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\]. How do you write the Lewis structures for these two compounds, and describe how measurement of dipole moments might be used to distinguish between them?
Answer
521.1k+ views
Hint:A Lewis Structure is a simplified representation of a molecule's valence shell electrons. It's used to demonstrate how electrons in a molecule are organised around individual atoms. Dipole moments are caused by variations in electronegativity and may occur between two ions in an ionic bond or between atoms in a covalent bond.
Complete answer:
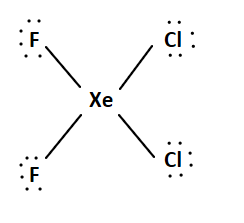
There are $32$ valence electrons in the trial structure.
\[{\text{1 Xe + 2 F + 2 Cl = 8 + 14 + 14 = 36 valence electrons}}\]
We add two-line pairs to the central atom since we have four extra electrons.
The Lewis structure is:
This system is an ${\text{A}}{{\text{X}}_4}{{\text{E}}_2}$system. It has an octahedral electron geometry. The F and Cl atoms will occupy the equatorial positions, while the bulky lone pairs will occupy the top and bottom axial positions. The molecule has a square planar geometry. We can arrange the atoms in two different ways.
The ${\text{F - Xe - F }}$and ${\text{Cl - Xe - Cl}}$ bond angles are 180 degrees in the trans isomer. The ${\text{Xe - Cl}}$ bond dipoles cancel the ${\text{Xe - F}}$ bond dipoles, and the ${\text{Xe - F}}$ bond dipoles cancel the ${\text{Xe - Cl}}$ bond dipoles. The trans isomer is nonpolar since there is no net dipole.
The \[{\text{Xe - F}}\] and ${\text{Xe - Cl}}$bond dipoles do not cancel in the cis-isomer. The molecule is polar in the cis isomer, which has a net dipole moment.
Note:
Cis isomers are molecules that have the same atom connectivity. They have side groups that are identical on the same side of a double bond. Molecules with identical side groups on opposite sides of a double bond are called trans isomers.
Complete answer:

There are $32$ valence electrons in the trial structure.
\[{\text{1 Xe + 2 F + 2 Cl = 8 + 14 + 14 = 36 valence electrons}}\]
We add two-line pairs to the central atom since we have four extra electrons.
The Lewis structure is:
This system is an ${\text{A}}{{\text{X}}_4}{{\text{E}}_2}$system. It has an octahedral electron geometry. The F and Cl atoms will occupy the equatorial positions, while the bulky lone pairs will occupy the top and bottom axial positions. The molecule has a square planar geometry. We can arrange the atoms in two different ways.
The ${\text{F - Xe - F }}$and ${\text{Cl - Xe - Cl}}$ bond angles are 180 degrees in the trans isomer. The ${\text{Xe - Cl}}$ bond dipoles cancel the ${\text{Xe - F}}$ bond dipoles, and the ${\text{Xe - F}}$ bond dipoles cancel the ${\text{Xe - Cl}}$ bond dipoles. The trans isomer is nonpolar since there is no net dipole.
The \[{\text{Xe - F}}\] and ${\text{Xe - Cl}}$bond dipoles do not cancel in the cis-isomer. The molecule is polar in the cis isomer, which has a net dipole moment.
Note:
Cis isomers are molecules that have the same atom connectivity. They have side groups that are identical on the same side of a double bond. Molecules with identical side groups on opposite sides of a double bond are called trans isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




