
What is the total number of unpaired electrons in Chromium?
A.$2$
B.$3$
C.$4$
D.$6$
Answer
539.4k+ views
Hint:We are that unpaired electrons are those electrons that are single which means they are not paired in the orbital of the subshell. To know the number of unpaired electrons in an atom or a molecule, it is important to know the electronic configuration of that atom or molecule. Chromium has a symbol $Cr$ and atomic number $24$ .
Complete step-by-step answer:We know that unpaired electrons are those electrons that are single which means they are not paired in the orbital of the subshell. To know the number of unpaired electrons in an atom or a molecule, it is important to know the electronic configuration of that atom or molecule. Now we know that Chromium is a $d$ block element and is called a transition metal. Transition elements have partially filled $d$ and $f$ subshells. They remain between $s$ and $p$ block elements. Chromium has a symbol $Cr$ and atomic number $24$ .
Now, according to Aufbau’s principle, Hunds’ rule, and Pauli’s Exclusion principle, the electronic configuration of $Cr$ is
$[Ar]4{s^2}3{d^4}$
But Chromium is an exception, it does not follow Aufbau’s principle and thus the actual configuration is
$[Ar]4{s^1}3{d^5}$
This exception arises because the electron moves from $4s$ subshell to have a half-filled $3d$ subshell. This gives the atom more stability. A half-filled subshell has more stability than a partially filled subshell. Now if we count the number of unpaired electrons from the diagram
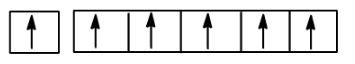
So the unpaired electrons are $6$
Thus the correct option is $D$.
Note:It is important to note that other elements also have exceptions when it comes to giving their electronic configuration. One such exception is copper, it also doesn’t follow Afbau’s principle. It has an electronic configuration $[Ar]4{s^1}3{d^{10}}$ .
Complete step-by-step answer:We know that unpaired electrons are those electrons that are single which means they are not paired in the orbital of the subshell. To know the number of unpaired electrons in an atom or a molecule, it is important to know the electronic configuration of that atom or molecule. Now we know that Chromium is a $d$ block element and is called a transition metal. Transition elements have partially filled $d$ and $f$ subshells. They remain between $s$ and $p$ block elements. Chromium has a symbol $Cr$ and atomic number $24$ .
Now, according to Aufbau’s principle, Hunds’ rule, and Pauli’s Exclusion principle, the electronic configuration of $Cr$ is
$[Ar]4{s^2}3{d^4}$
But Chromium is an exception, it does not follow Aufbau’s principle and thus the actual configuration is
$[Ar]4{s^1}3{d^5}$
This exception arises because the electron moves from $4s$ subshell to have a half-filled $3d$ subshell. This gives the atom more stability. A half-filled subshell has more stability than a partially filled subshell. Now if we count the number of unpaired electrons from the diagram

So the unpaired electrons are $6$
Thus the correct option is $D$.
Note:It is important to note that other elements also have exceptions when it comes to giving their electronic configuration. One such exception is copper, it also doesn’t follow Afbau’s principle. It has an electronic configuration $[Ar]4{s^1}3{d^{10}}$ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




