
Total number of geometrical isomers for the complex $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$is: __
Answer
581.7k+ views
Hint: The isomerism in which the position of the ligands in the space differs is known as geometrical isomerism. The square planar complexes show geometrical isomers. These complexes can be of various types such as$\text{ M}{{\text{A}}_{\text{2}}}{{\text{B}}_{\text{2}}}\text{ , M}{{\text{A}}_{\text{2}}}\text{BC , MAB}{{\text{C}}_{\text{2}}}\text{ ,MABCD }$. The number of isomers depends on the number of different positions acquired by the ligand.
Complete answer:
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy the positions either adjacent to one another. These are referred to as cis-form (ligands occupy adjacent positions) and transforms (ligands occupy opposite positions). This type of isomerism is, therefore also referred to as the cis-trans isomerism.
This type of isomerism is very common in coordination compounds. This is due to different coordination numbers varying from 2 to 9, commonly encountered in these compounds.
We have given the coordination compound of ruthenium$\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$. It is coordination compounds, such that the central atom $\text{ Rh }$ is bonded to four different ligands: $\text{ Cl }$ ,$\text{ CO }$ , $\text{ PP}{{\text{h}}_{\text{3}}}\text{ }$ and $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$ .
Since $\text{ Rh }$is bonded with four ligands, it must be a square planar complex. The bonded groups are different thus its general representation is, $\text{ MABCD }$ , where A, B, C, and D are ligands.
The square planar complexes of $\text{ MABCD }$type structure show three isomers. the structures of these isomers can be written by fixing a position of a ligand (let say A) and placing other and D ligands trans to it.
To determine the geometrical isomers of $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$let's fix the position of $\text{ Cl }$the ligand and place the other ligands trans to it. The geometrical isomers for the $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$are as shown in the figure.
Thus, here we conclude that the coordination complex $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$has three isomers.
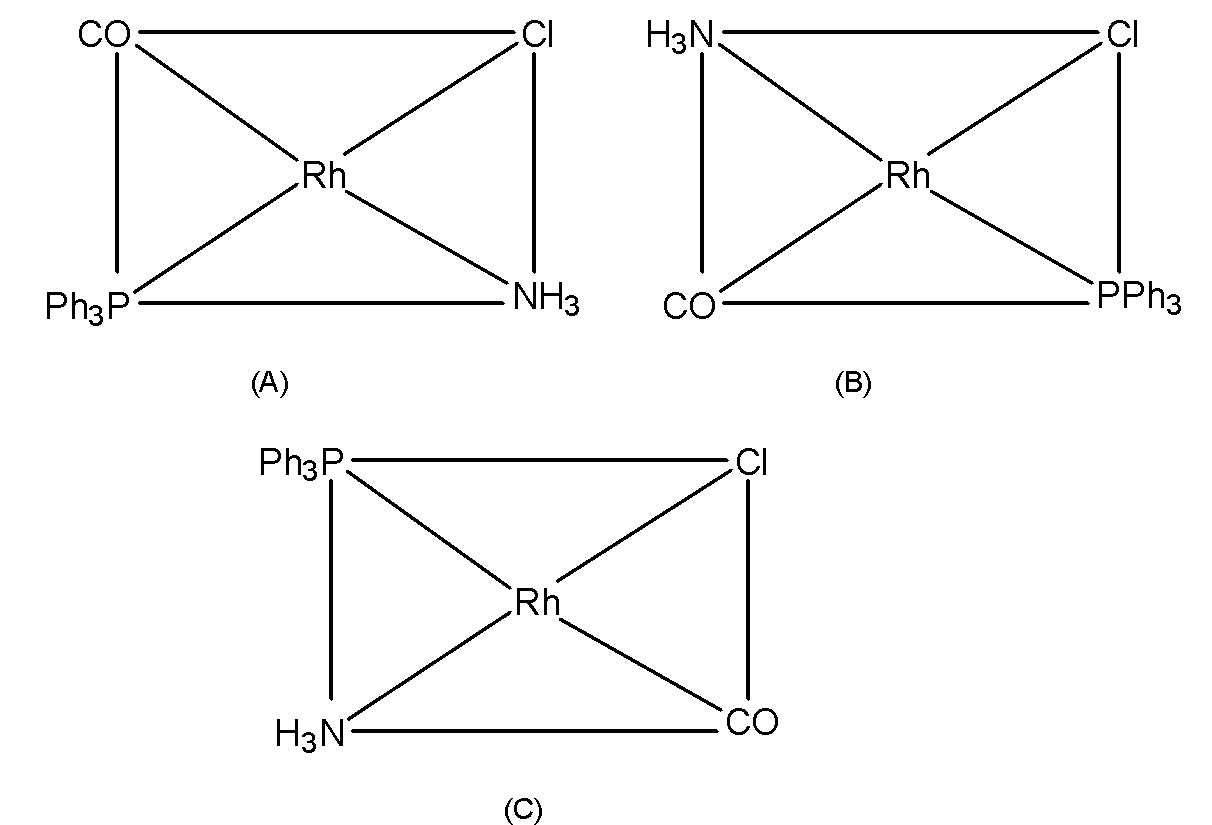
Here, (A), (B), and (C) are geometrical isomers of the complex.
Note:
Note that, we can draw forth geometrical isomers.IN which the Chloride ligand is trans to the $\text{ PP}{{\text{h}}_{\text{3}}}\text{ }$ligand which is identical to the (A) form. Thus, only three isomers are possible for the $\text{ MABCD }$type of complex. The geometrical isomers are not possible in tetrahedral complexes as all the positions are adjacent to each other in these complexes. Thus, geometrical isomerism is strictly related to the square planar complexes.
Complete answer:
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy the positions either adjacent to one another. These are referred to as cis-form (ligands occupy adjacent positions) and transforms (ligands occupy opposite positions). This type of isomerism is, therefore also referred to as the cis-trans isomerism.
This type of isomerism is very common in coordination compounds. This is due to different coordination numbers varying from 2 to 9, commonly encountered in these compounds.
We have given the coordination compound of ruthenium$\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$. It is coordination compounds, such that the central atom $\text{ Rh }$ is bonded to four different ligands: $\text{ Cl }$ ,$\text{ CO }$ , $\text{ PP}{{\text{h}}_{\text{3}}}\text{ }$ and $\text{ N}{{\text{H}}_{\text{3}}}\text{ }$ .
Since $\text{ Rh }$is bonded with four ligands, it must be a square planar complex. The bonded groups are different thus its general representation is, $\text{ MABCD }$ , where A, B, C, and D are ligands.
The square planar complexes of $\text{ MABCD }$type structure show three isomers. the structures of these isomers can be written by fixing a position of a ligand (let say A) and placing other and D ligands trans to it.
To determine the geometrical isomers of $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$let's fix the position of $\text{ Cl }$the ligand and place the other ligands trans to it. The geometrical isomers for the $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$are as shown in the figure.
Thus, here we conclude that the coordination complex $\text{ }\left[ \text{RhCl(CO)(PP}{{\text{h}}_{\text{3}}}\text{)(N}{{\text{H}}_{\text{3}}}\text{)} \right]\text{ }$has three isomers.

Here, (A), (B), and (C) are geometrical isomers of the complex.
Note:
Note that, we can draw forth geometrical isomers.IN which the Chloride ligand is trans to the $\text{ PP}{{\text{h}}_{\text{3}}}\text{ }$ligand which is identical to the (A) form. Thus, only three isomers are possible for the $\text{ MABCD }$type of complex. The geometrical isomers are not possible in tetrahedral complexes as all the positions are adjacent to each other in these complexes. Thus, geometrical isomerism is strictly related to the square planar complexes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




