
The total number of isomeric bromo-chloro-fluoro- iodo propadiene is:
A. 5
B. 6
C. 7
D. 8
Answer
569.7k+ views
Hint: Isomers are polyatomic ions with the same number of elements present in the compound, just that their geometry changes. The isomers may or may not have the same physical or chemical properties.
Complete Solution :
If we look at the structure of the compound given in the question, then it is an ethene with three carbon chains and chloro, bromo, iodo and fluoro group is attached to it.
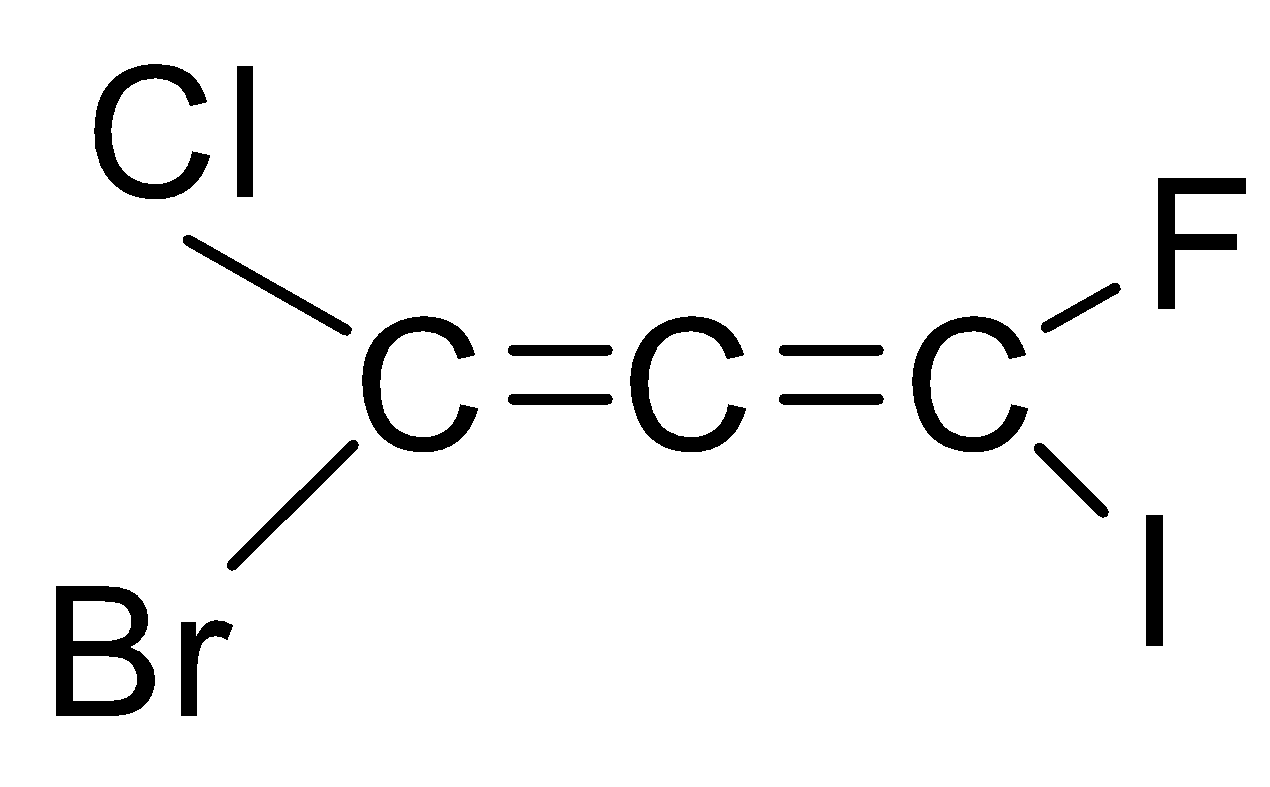
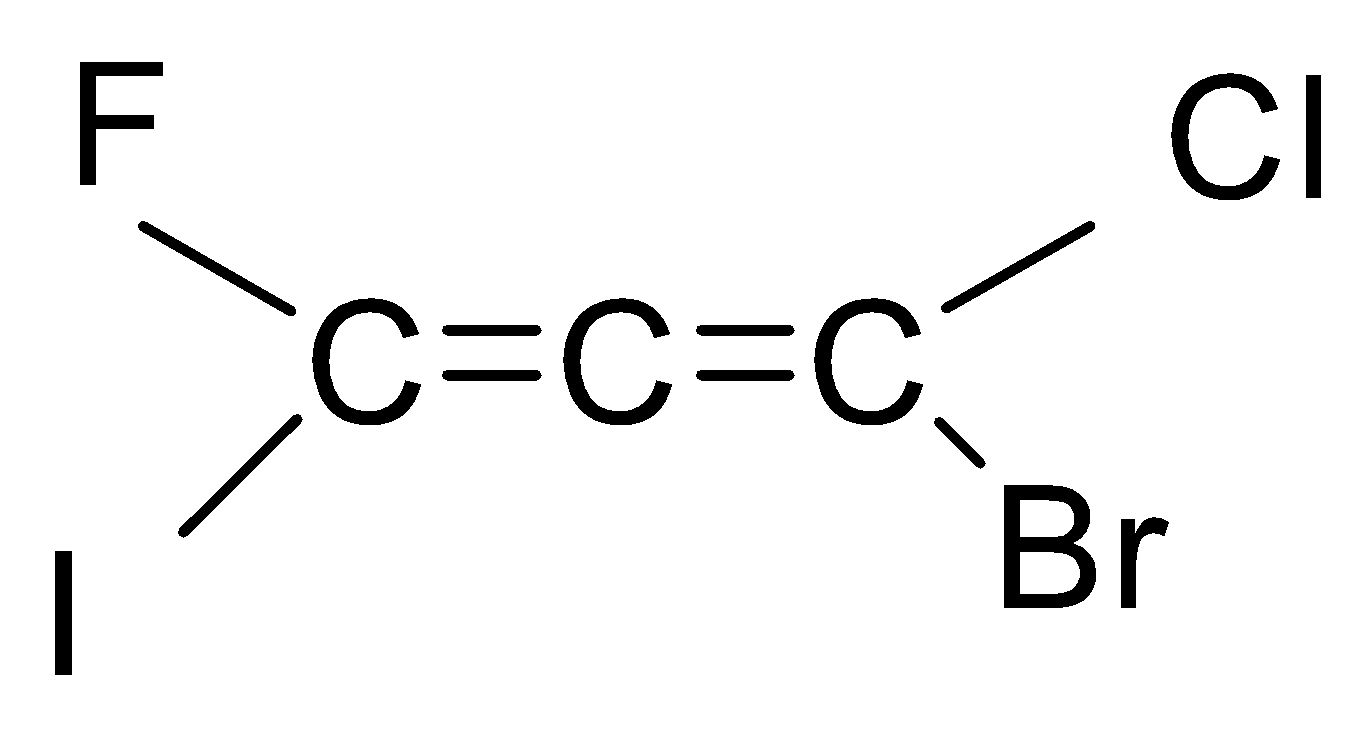
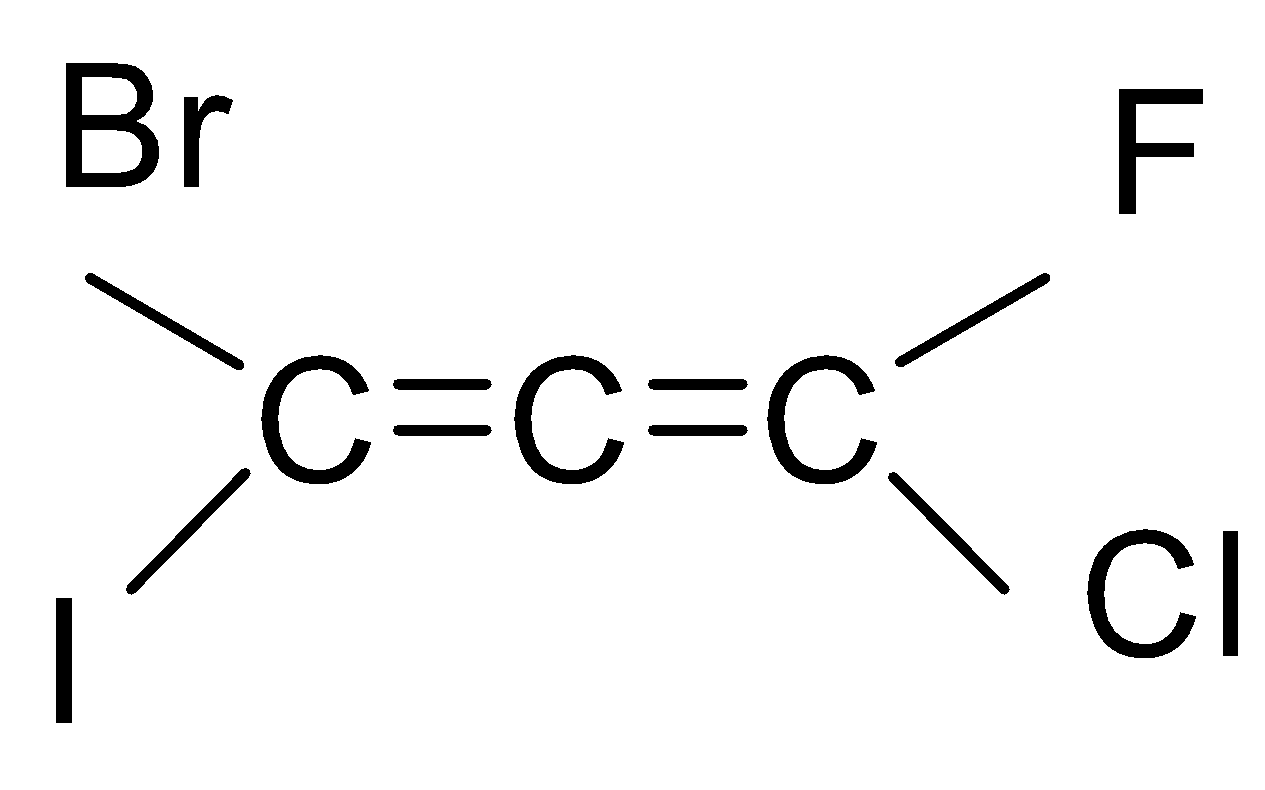
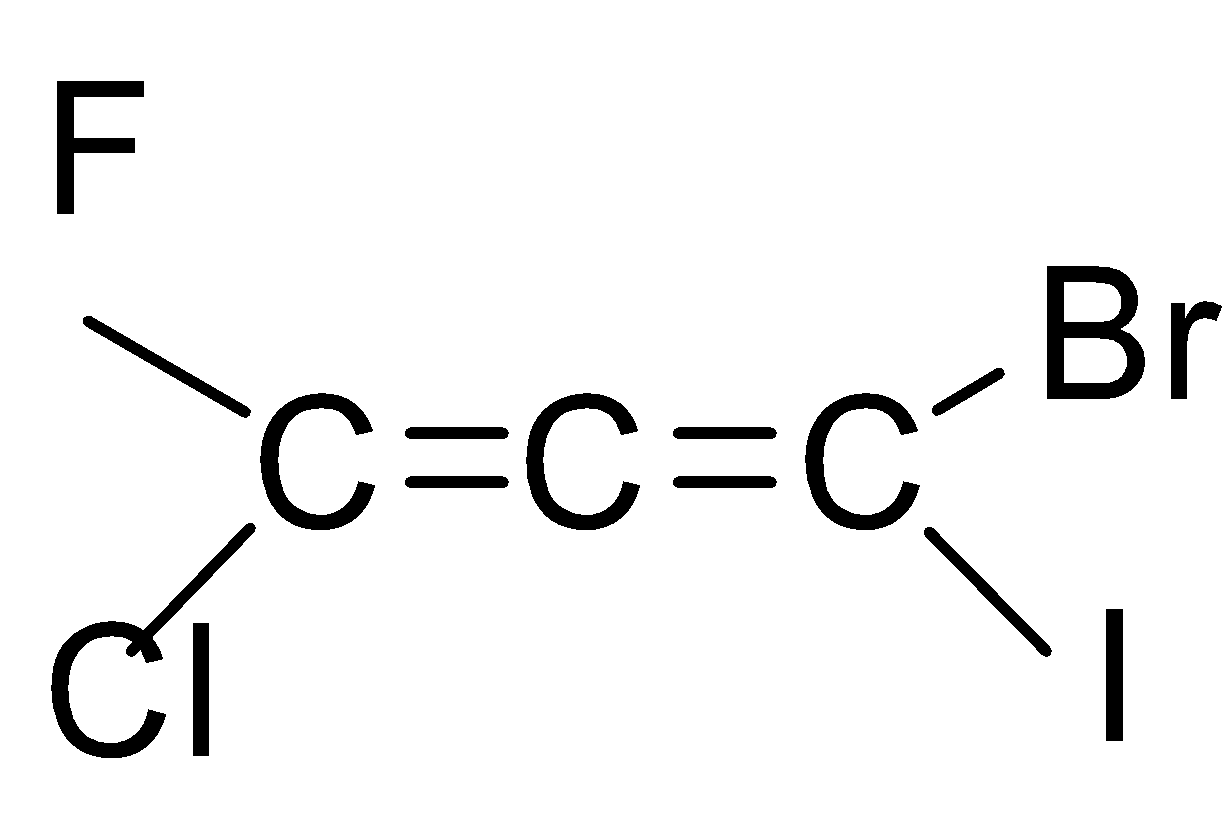
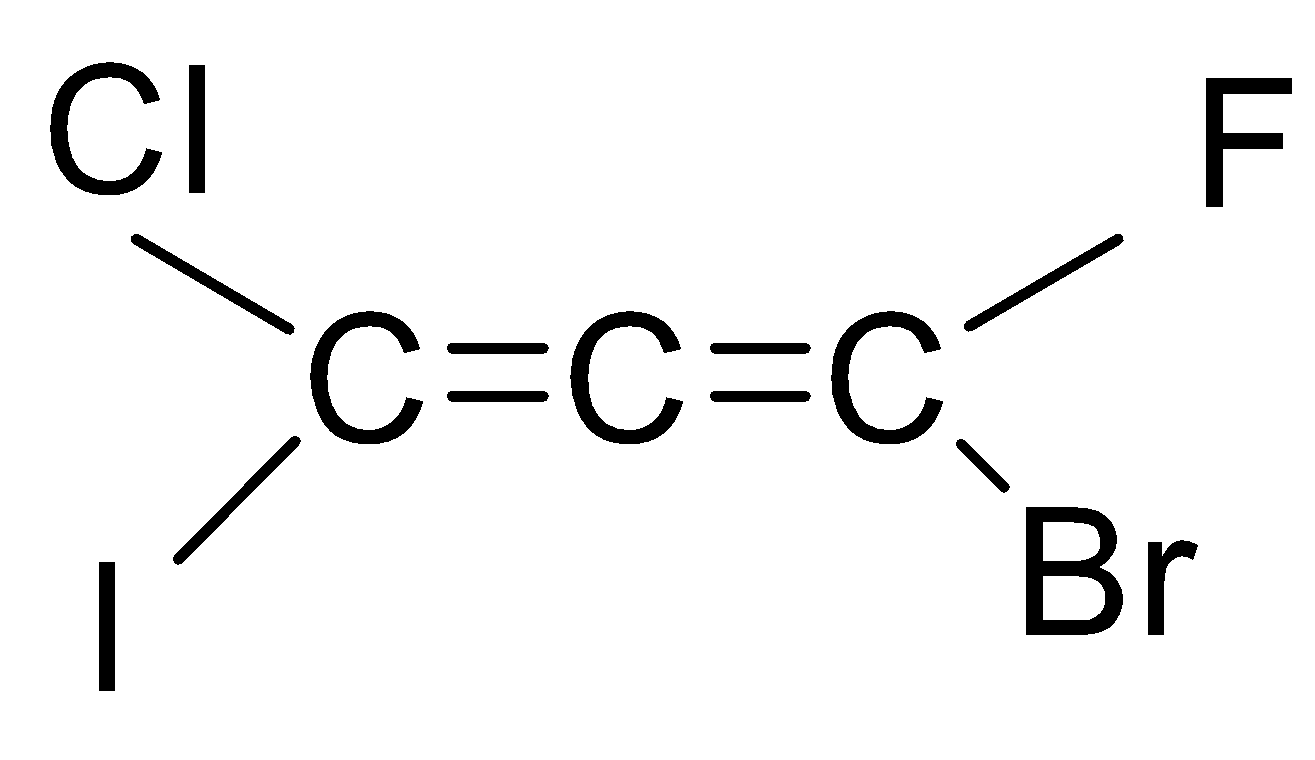
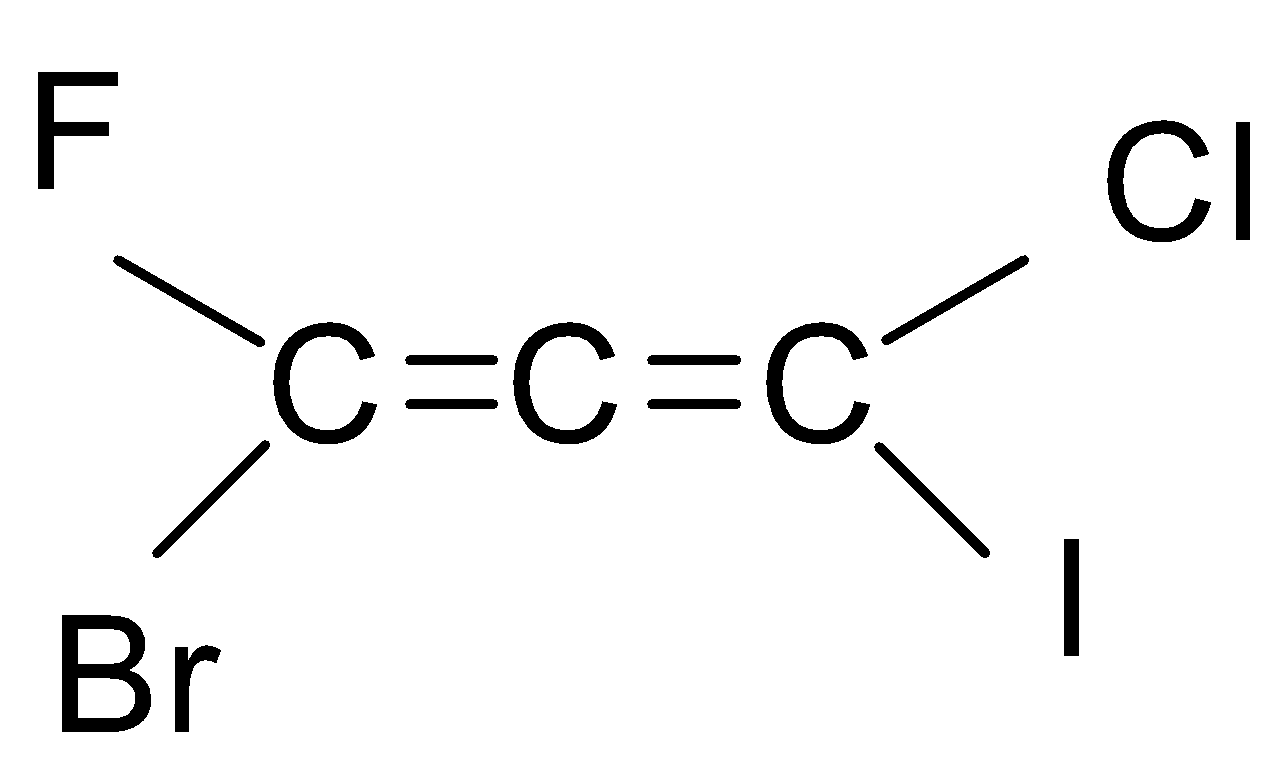
As you can see that there are six types of isomers possible of bromo-chloro-fluoro-iodo propadiene. These isomers are mirror images of each other that means it is a pair of d and l isomers which are optically active. Optically active isomers mean that there are the same no. of atoms and just the spatial arrangement of the atoms are different around the diene. These are all non-superimposable mirror images of each other also called enantiomers. These pairs form a racemic mixture which contains a ratio of d and l isomers both. So, the correct answer is “Option B”.
Note: Here the d and l does not represent dextro and laevo it simply means the orientation of plane polarized light. If the compound rotates the light in clockwise form, then it is the d-form (+) and if it rotates the light in an anti-clockwise manner, then it is known as l-form (-). With the help of combination of these two racemic mixtures are formed.
Complete Solution :
If we look at the structure of the compound given in the question, then it is an ethene with three carbon chains and chloro, bromo, iodo and fluoro group is attached to it.



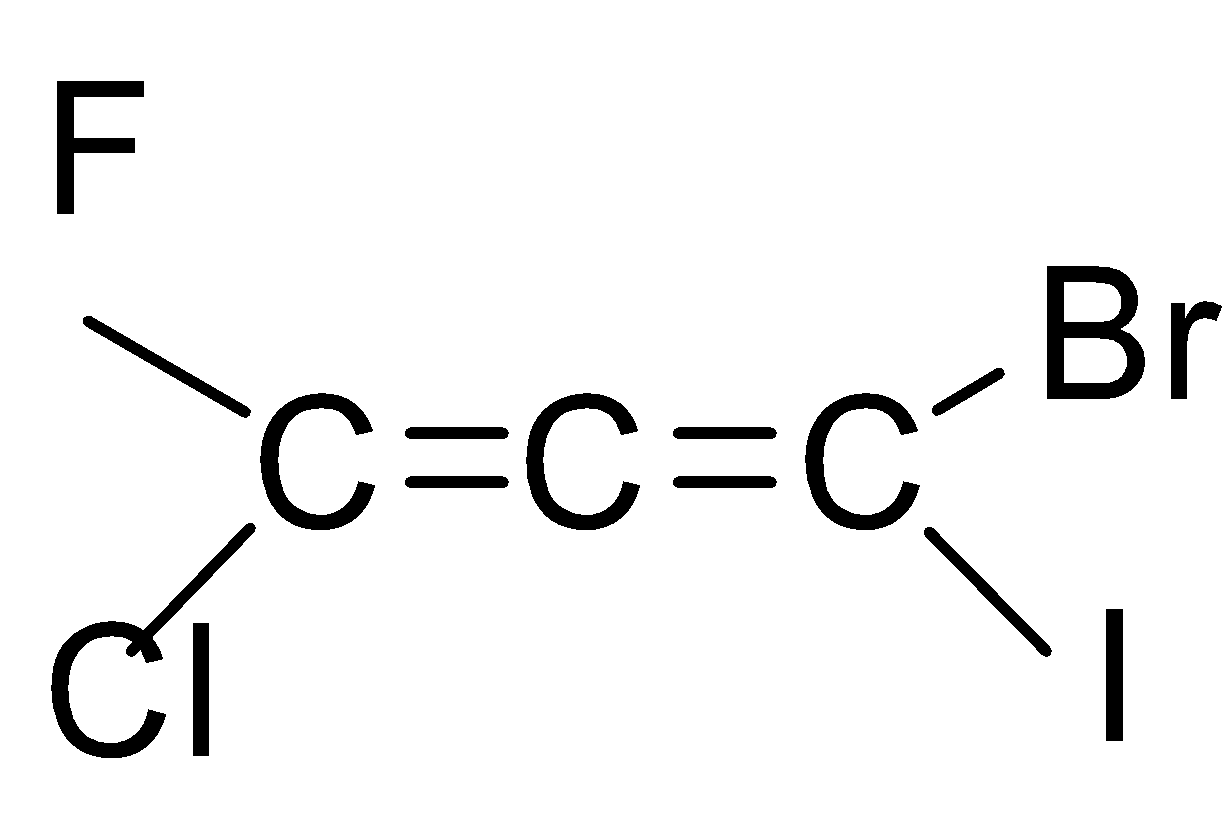


As you can see that there are six types of isomers possible of bromo-chloro-fluoro-iodo propadiene. These isomers are mirror images of each other that means it is a pair of d and l isomers which are optically active. Optically active isomers mean that there are the same no. of atoms and just the spatial arrangement of the atoms are different around the diene. These are all non-superimposable mirror images of each other also called enantiomers. These pairs form a racemic mixture which contains a ratio of d and l isomers both. So, the correct answer is “Option B”.
Note: Here the d and l does not represent dextro and laevo it simply means the orientation of plane polarized light. If the compound rotates the light in clockwise form, then it is the d-form (+) and if it rotates the light in an anti-clockwise manner, then it is known as l-form (-). With the help of combination of these two racemic mixtures are formed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




