
The term anomers of glucose refers to:
(A) isomers of glucose that differ in configurations at carbon 1 and 4
(B) a mixture of (D) glucose and (L) glucose
(C) enantiomers of glucose
(D) isomers of glucose that differ in configuration at carbon 1
Answer
584.1k+ views
Hint: It is important to identify the type of isomerism exhibited by a pair of anomers i.e. structural or stereoisomerism. Identifying the distinguishing factor between the pair of isomers. With this you can identify the reason why they exhibit isomerism and what kind of isomerism as well.
Complete step-by-step answer:
Isomerism is the phenomenon in which two or more compounds have the same chemical formula but differ in chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers i.e. they exhibit isomerism.
Isomerism is of two types namely, Structural isomerism and stereoisomerism.
In stereoisomerism, the compounds have the same chemical formula but differ in their respective orientations of the atoms belonging to the compound in a 3D space.
The types of stereoisomerism are:
- Geometrical
- Optical
Anomers are cyclic monosaccharides, differing from each other in the configuration of C-1 carbon or C-2 carbon. For aldoses, it is C-1 and C-2 for ketoses.
The distinguishing carbon atom is called anomeric carbon or anomeric center.
We will now draw the pair of anomers for the monosaccharide, glucose.
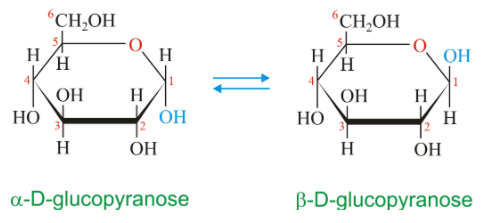
Therefore, the correct answer is option (D).
Note: It is important to know that not all epimers are the same as anomers. Epimers are a pair of isomers with different configuration of atoms about any chiral carbon center. However, anomers differ in configuration either at C-1 or C-2 only. Thus, Anomers are a subset of epimers.
Complete step-by-step answer:
Isomerism is the phenomenon in which two or more compounds have the same chemical formula but differ in chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers i.e. they exhibit isomerism.
Isomerism is of two types namely, Structural isomerism and stereoisomerism.
In stereoisomerism, the compounds have the same chemical formula but differ in their respective orientations of the atoms belonging to the compound in a 3D space.
The types of stereoisomerism are:
- Geometrical
- Optical
Anomers are cyclic monosaccharides, differing from each other in the configuration of C-1 carbon or C-2 carbon. For aldoses, it is C-1 and C-2 for ketoses.
The distinguishing carbon atom is called anomeric carbon or anomeric center.
We will now draw the pair of anomers for the monosaccharide, glucose.

Therefore, the correct answer is option (D).
Note: It is important to know that not all epimers are the same as anomers. Epimers are a pair of isomers with different configuration of atoms about any chiral carbon center. However, anomers differ in configuration either at C-1 or C-2 only. Thus, Anomers are a subset of epimers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




