
The structure of toluene is?
A.
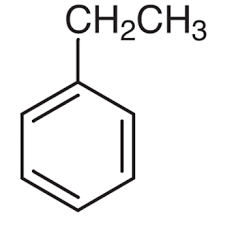
B.
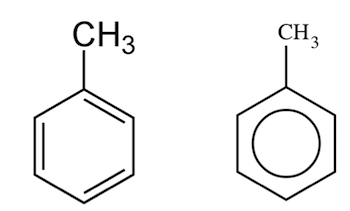
C.
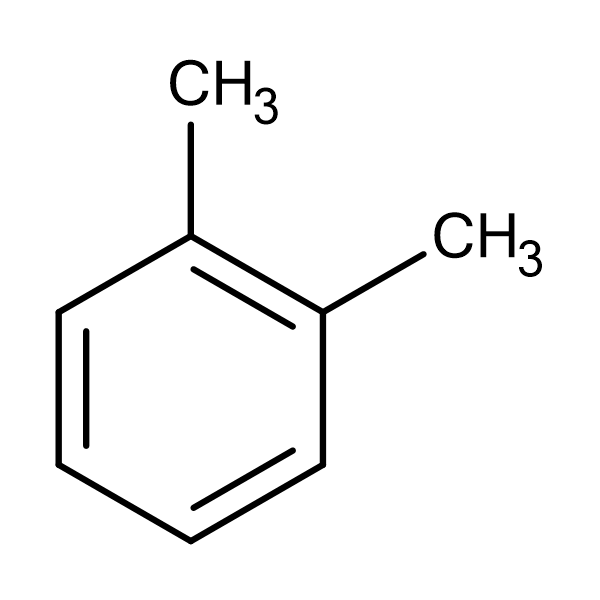
D.
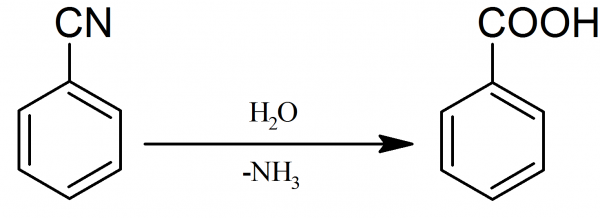




Answer
609.6k+ views
Hint: Toluene is a mono-substituted benzene derivative and it is more reactive than benzene toward electrophiles. We can use properties to identify the correct structure.
Complete step by step answer:
Toluene is an aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners.
It is a mono-substituted benzene derivative, consisting of a methyl group attached to a phenyl group. As such, its IUPAC systematic name is methylbenzene.
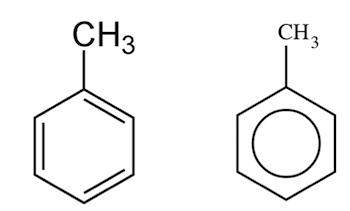
Which is given in option B .
We can also identify structures provided in other options too.
The structure in option A, is of ethylbenzene. It is also a mono-substituted benzene derivative.
The structure in option C, is of 1,2-dimethylbenzene. It is a di-substituted benzene derivative.
The structure in option D, is of benzoic acid. It is also a mono-substituted benzene derivative.
Thus, the correct option is B.
Additional information: Toluene is used as the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue.
Toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm.
Note: Toluene reacts as a normal aromatic hydrocarbon in electrophilic aromatic substitution. Because the methyl group has greater electron-releasing properties than a hydrogen atom in the same position, toluene is more reactive than benzene toward electrophiles.
Complete step by step answer:
Toluene is an aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners.
It is a mono-substituted benzene derivative, consisting of a methyl group attached to a phenyl group. As such, its IUPAC systematic name is methylbenzene.

Which is given in option B .
We can also identify structures provided in other options too.
The structure in option A, is of ethylbenzene. It is also a mono-substituted benzene derivative.
The structure in option C, is of 1,2-dimethylbenzene. It is a di-substituted benzene derivative.
The structure in option D, is of benzoic acid. It is also a mono-substituted benzene derivative.
Thus, the correct option is B.
Additional information: Toluene is used as the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue.
Toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm.
Note: Toluene reacts as a normal aromatic hydrocarbon in electrophilic aromatic substitution. Because the methyl group has greater electron-releasing properties than a hydrogen atom in the same position, toluene is more reactive than benzene toward electrophiles.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




